

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholinesterase (Equus caballus (Horse)) | BDBM50210854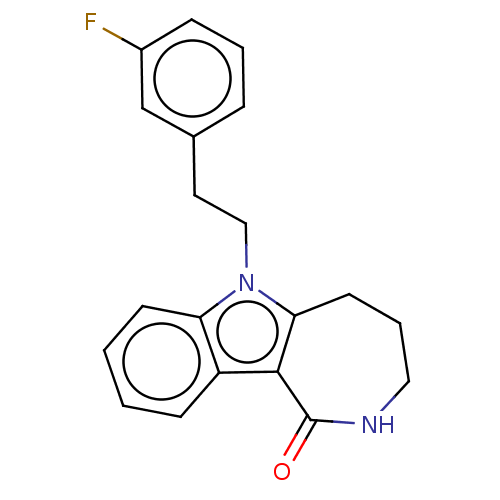 (CHEMBL3960040) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari"A. Moro" Curated by ChEMBL | Assay Description Competitive inhibition of horse serum BChE in presence of varying levels of butyrylthiocholine iodide substrate by Lineweaver-burk plot method | Eur J Med Chem 125: 288-298 (2017) Article DOI: 10.1016/j.ejmech.2016.09.037 BindingDB Entry DOI: 10.7270/Q2G44S9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50515472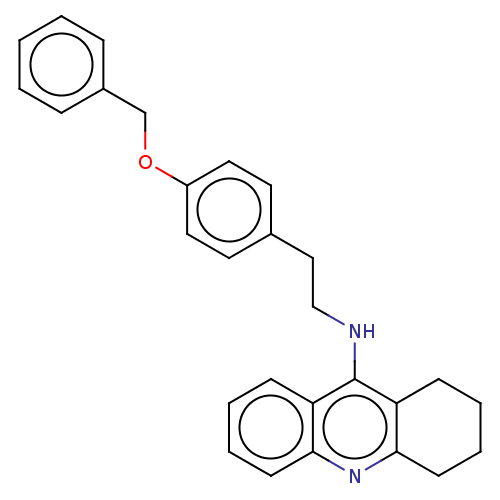 (CHEMBL4469822) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE assessed as dissociation constant for enzyme-substrate-inhibitor complex using butyrylthiocholine iodide as substrat... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.07.053 BindingDB Entry DOI: 10.7270/Q23R0X60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50515454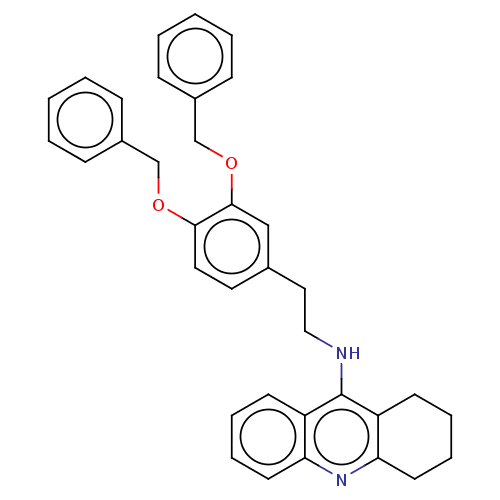 (CHEMBL4550977) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE assessed as dissociation constant for enzyme-substrate-inhibitor complex using butyrylthiocholine iodide as substrat... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.07.053 BindingDB Entry DOI: 10.7270/Q23R0X60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50515453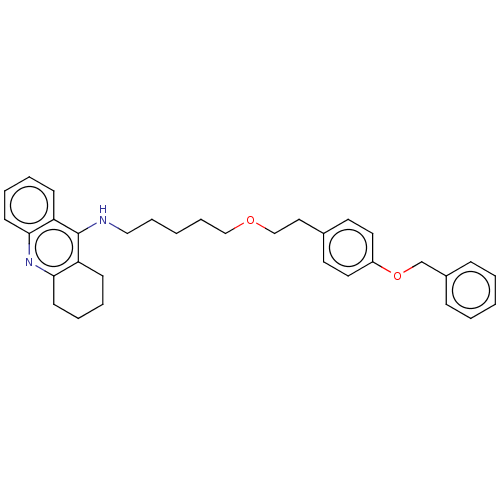 (CHEMBL4588525) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE assessed as dissociation constant for enzyme-substrate-inhibitor complex using butyrylthiocholine iodide as substrat... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.07.053 BindingDB Entry DOI: 10.7270/Q23R0X60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50515473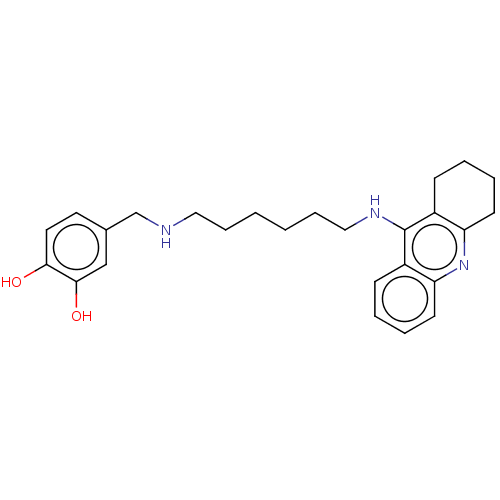 (CHEMBL4572757) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE assessed as dissociation constant for enzyme-substrate-inhibitor complex using butyrylthiocholine iodide as substrat... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.07.053 BindingDB Entry DOI: 10.7270/Q23R0X60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50433313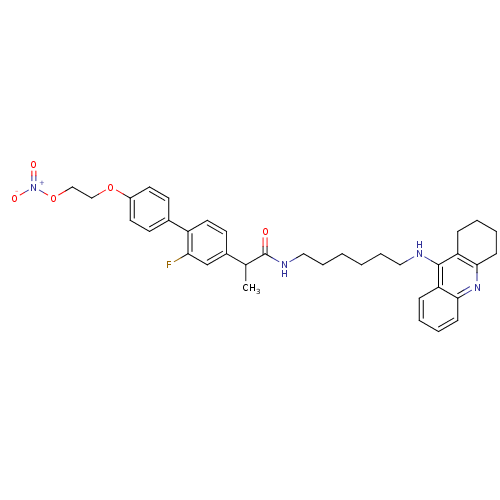 (CHEMBL2376474) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BChE by Lineweaver Burk reciprocal plot analysis in presence of acetylcholine | Bioorg Med Chem 21: 2462-70 (2013) Article DOI: 10.1016/j.bmc.2013.03.005 BindingDB Entry DOI: 10.7270/Q2319X82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50515465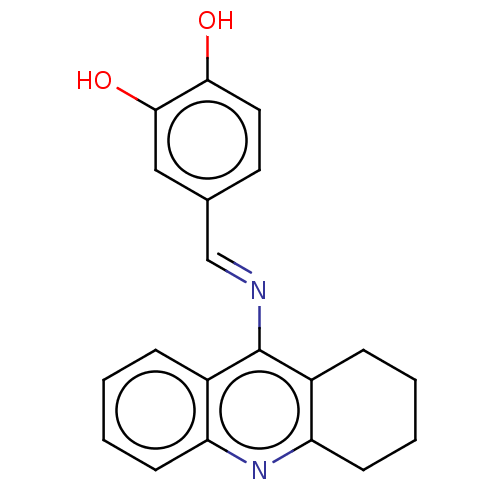 (CHEMBL4536715) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE assessed as dissociation constant for enzyme-substrate-inhibitor complex using butyrylthiocholine iodide as substrat... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.07.053 BindingDB Entry DOI: 10.7270/Q23R0X60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50515453 (CHEMBL4588525) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE assessed as dissociation constant for enzyme-inhibitor complex using butyrylthiocholine iodide as substrate measured... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.07.053 BindingDB Entry DOI: 10.7270/Q23R0X60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50515470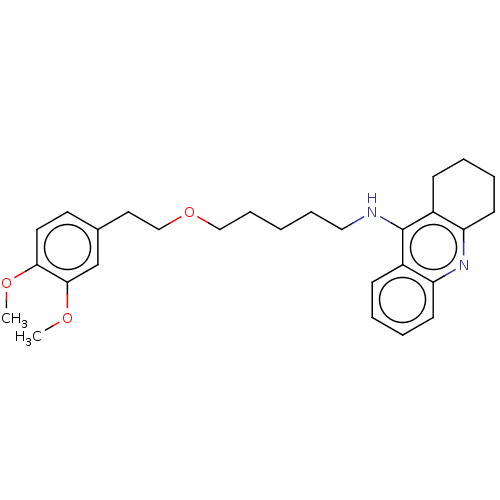 (CHEMBL4555120) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE assessed as dissociation constant for enzyme-substrate-inhibitor complex using butyrylthiocholine iodide as substrat... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.07.053 BindingDB Entry DOI: 10.7270/Q23R0X60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8963 (CHEMBL32823 | Homodimeric Tacrine Analog 3b | N-[7...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Inhibition of horse BuChE | J Med Chem 51: 3154-70 (2008) Article DOI: 10.1021/jm701253t BindingDB Entry DOI: 10.7270/Q22Z16D4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50515459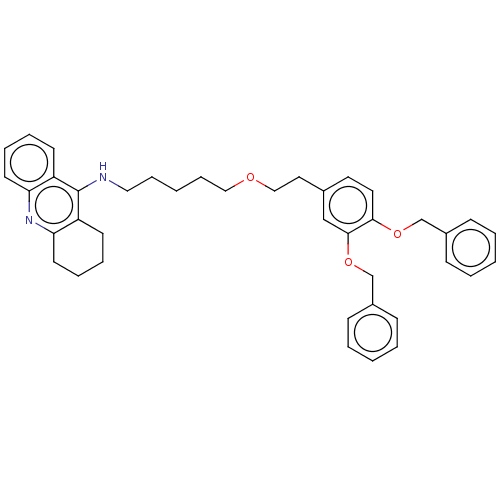 (CHEMBL4545701) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE assessed as dissociation constant for enzyme-substrate-inhibitor complex using butyrylthiocholine iodide as substrat... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.07.053 BindingDB Entry DOI: 10.7270/Q23R0X60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50515460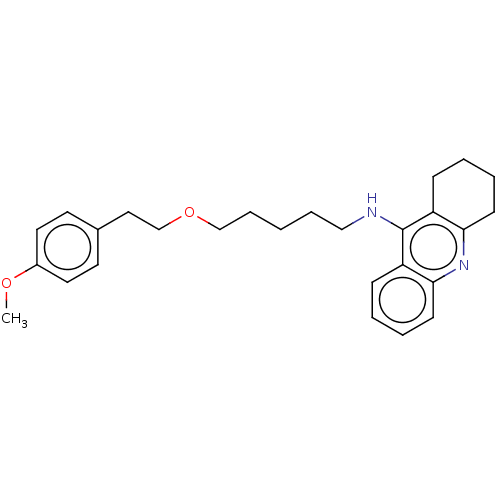 (CHEMBL4475228) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE assessed as dissociation constant for enzyme-substrate-inhibitor complex using butyrylthiocholine iodide as substrat... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.07.053 BindingDB Entry DOI: 10.7270/Q23R0X60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50515468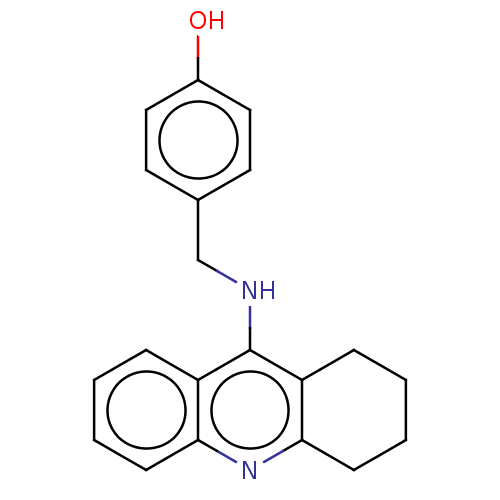 (CHEMBL4483710) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE assessed as dissociation constant for enzyme-substrate-inhibitor complex using butyrylthiocholine iodide as substrat... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.07.053 BindingDB Entry DOI: 10.7270/Q23R0X60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50515461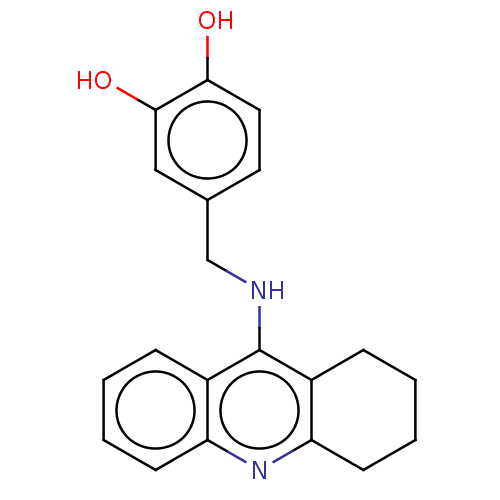 (CHEMBL4535585) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE assessed as dissociation constant for enzyme-substrate-inhibitor complex using butyrylthiocholine iodide as substrat... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.07.053 BindingDB Entry DOI: 10.7270/Q23R0X60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8974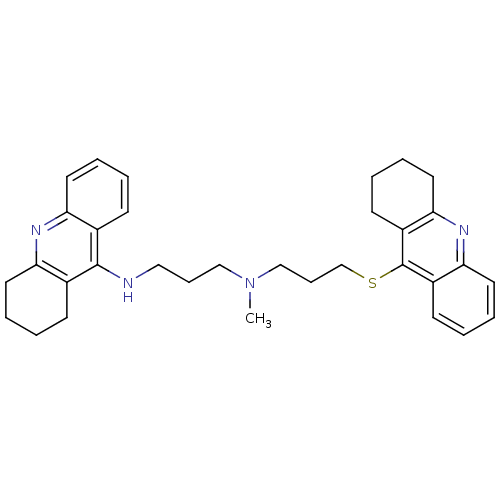 (CHEMBL367067 | N-Methyl-N-(1,2,3,4-tetrahydroacrid...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Inhibition of horse BuChE | J Med Chem 51: 3154-70 (2008) Article DOI: 10.1021/jm701253t BindingDB Entry DOI: 10.7270/Q22Z16D4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50515471 (CHEMBL4531167) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE assessed as dissociation constant for enzyme-substrate-inhibitor complex using butyrylthiocholine iodide as substrat... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.07.053 BindingDB Entry DOI: 10.7270/Q23R0X60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50515459 (CHEMBL4545701) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE assessed as dissociation constant for enzyme-inhibitor complex using butyrylthiocholine iodide as substrate measured... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.07.053 BindingDB Entry DOI: 10.7270/Q23R0X60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50515469 (CHEMBL4521755) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE assessed as dissociation constant for enzyme-substrate-inhibitor complex using butyrylthiocholine iodide as substrat... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.07.053 BindingDB Entry DOI: 10.7270/Q23R0X60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM199186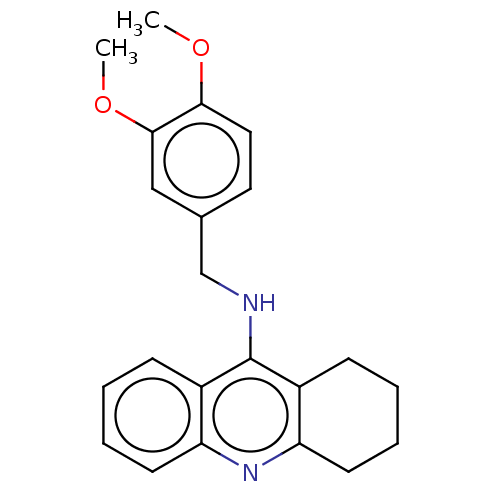 (N-(3,4-Dimethoxybenzyl)-1,2,3,4-tetrahydroacridin-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE assessed as dissociation constant for enzyme-substrate-inhibitor complex using butyrylthiocholine iodide as substrat... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.07.053 BindingDB Entry DOI: 10.7270/Q23R0X60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50515470 (CHEMBL4555120) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE assessed as dissociation constant for enzyme-inhibitor complex using butyrylthiocholine iodide as substrate measured... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.07.053 BindingDB Entry DOI: 10.7270/Q23R0X60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50515473 (CHEMBL4572757) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE assessed as dissociation constant for enzyme-inhibitor complex using butyrylthiocholine iodide as substrate measured... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.07.053 BindingDB Entry DOI: 10.7270/Q23R0X60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50515462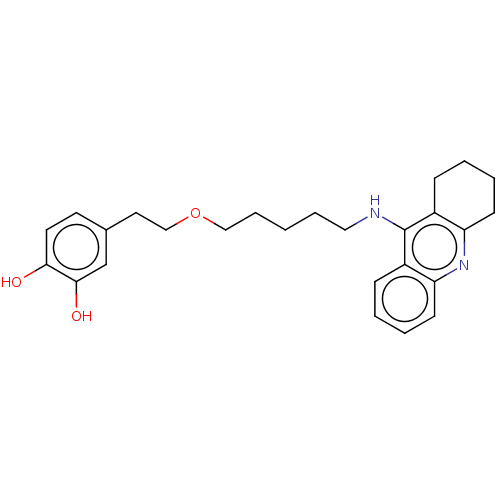 (CHEMBL4589250) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE assessed as dissociation constant for enzyme-substrate-inhibitor complex using butyrylthiocholine iodide as substrat... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.07.053 BindingDB Entry DOI: 10.7270/Q23R0X60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50345196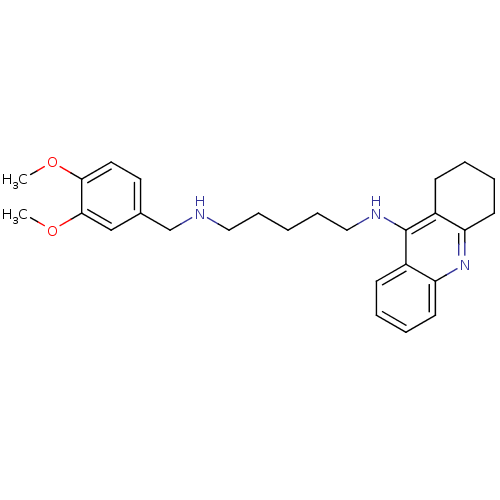 (CHEMBL1783191 | N1-(3,4-Dimethoxybenzyl)-N5-(1,2,3...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE assessed as dissociation constant for enzyme-substrate-inhibitor complex using butyrylthiocholine iodide as substrat... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.07.053 BindingDB Entry DOI: 10.7270/Q23R0X60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50234798 (CHEMBL4091899) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition meas... | Eur J Med Chem 126: 652-668 (2017) Article DOI: 10.1016/j.ejmech.2016.11.056 BindingDB Entry DOI: 10.7270/Q2X0698K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50456710 (CHEMBL4213722) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Non-competitive inhibition of equine serum BuChE assessed as enzyme-inhibitor complex using p-nitrophenyl butyrate as substrate by Lineweaver-Burk pl... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50456710 (CHEMBL4213722) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Non-competitive inhibition of equine serum BuChE assessed as enzyme-substrate-inhibitor complex using p-nitrophenyl butyrate as substrate by Lineweav... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50515465 (CHEMBL4536715) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE assessed as dissociation constant for enzyme-inhibitor complex using butyrylthiocholine iodide as substrate measured... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.07.053 BindingDB Entry DOI: 10.7270/Q23R0X60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50515468 (CHEMBL4483710) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE assessed as dissociation constant for enzyme-inhibitor complex using butyrylthiocholine iodide as substrate measured... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.07.053 BindingDB Entry DOI: 10.7270/Q23R0X60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50392999 (CHEMBL2152545) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Time dependent inhibition of equine serum BChE assessed as stability constants of inhibitor-enzyme complex using acetylthiocholine as substrate after... | ACS Med Chem Lett 3: 914-919 (2012) Article DOI: 10.1021/ml3001825 BindingDB Entry DOI: 10.7270/Q2P27079 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50456701 (CHEMBL4218651) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Mixed-type inhibition of equine serum BuChE assessed as enzyme-substrate-inhibitor complex using p-nitrophenyl butyrate as substrate by Lineweaver-Bu... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50515467 (CHEMBL4439554) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE assessed as dissociation constant for enzyme-substrate-inhibitor complex using butyrylthiocholine iodide as substrat... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.07.053 BindingDB Entry DOI: 10.7270/Q23R0X60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BuChE assessed as enzyme-inhibitor complex using p-nitrophenyl butyrate as substrate by Lineweaver-Burk plot a... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE assessed as dissociation constant for enzyme-inhibitor complex using butyrylthiocholine iodide as substrate measured... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.07.053 BindingDB Entry DOI: 10.7270/Q23R0X60 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50515454 (CHEMBL4550977) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE assessed as dissociation constant for enzyme-inhibitor complex using butyrylthiocholine iodide as substrate measured... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.07.053 BindingDB Entry DOI: 10.7270/Q23R0X60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50515472 (CHEMBL4469822) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE assessed as dissociation constant for enzyme-inhibitor complex using butyrylthiocholine iodide as substrate measured... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.07.053 BindingDB Entry DOI: 10.7270/Q23R0X60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50555654 (CHEMBL4797435) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Mixed inhibition of equine serum BChE using butyrylthiolcholine iodide as substrate by Lineweaver-Burk plot analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112273 BindingDB Entry DOI: 10.7270/Q2T157BP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50515461 (CHEMBL4535585) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE assessed as dissociation constant for enzyme-inhibitor complex using butyrylthiocholine iodide as substrate measured... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.07.053 BindingDB Entry DOI: 10.7270/Q23R0X60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50515464 (CHEMBL3182739) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE assessed as dissociation constant for enzyme-substrate-inhibitor complex using butyrylthiocholine iodide as substrat... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.07.053 BindingDB Entry DOI: 10.7270/Q23R0X60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50456709 (CHEMBL4205454) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Non-competitive inhibition of equine serum BuChE assessed as enzyme-inhibitor complex using p-nitrophenyl butyrate as substrate by Lineweaver-Burk pl... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50515460 (CHEMBL4475228) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE assessed as dissociation constant for enzyme-inhibitor complex using butyrylthiocholine iodide as substrate measured... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.07.053 BindingDB Entry DOI: 10.7270/Q23R0X60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50456709 (CHEMBL4205454) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Non-competitive inhibition of equine serum BuChE assessed as enzyme-substrate-inhibitor complex using p-nitrophenyl butyrate as substrate by Lineweav... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50197240 (CHEMBL248088 | KYS-05080 | N-Benzyl-2-{3-biphenyl-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Mixed/noncompetitive inhibition of equine serum BCHE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addi... | Bioorg Med Chem Lett 27: 1179-1185 (2017) Article DOI: 10.1016/j.bmcl.2017.01.068 BindingDB Entry DOI: 10.7270/Q2P271CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50345196 (CHEMBL1783191 | N1-(3,4-Dimethoxybenzyl)-N5-(1,2,3...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE assessed as dissociation constant for enzyme-inhibitor complex using butyrylthiocholine iodide as substrate measured... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.07.053 BindingDB Entry DOI: 10.7270/Q23R0X60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM199186 (N-(3,4-Dimethoxybenzyl)-1,2,3,4-tetrahydroacridin-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE assessed as dissociation constant for enzyme-inhibitor complex using butyrylthiocholine iodide as substrate measured... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.07.053 BindingDB Entry DOI: 10.7270/Q23R0X60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50515456 (CHEMBL4564873) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE assessed as dissociation constant for enzyme-substrate-inhibitor complex using butyrylthiocholine iodide as substrat... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.07.053 BindingDB Entry DOI: 10.7270/Q23R0X60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50456701 (CHEMBL4218651) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Mixed-type inhibition of equine serum BuChE assessed as enzyme-inhibitor complex using p-nitrophenyl butyrate as substrate by Lineweaver-Burk plot an... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50456702 (CHEMBL4213042) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Mixed-type inhibition of equine serum BuChE assessed as enzyme-substrate-inhibitor complex using p-nitrophenyl butyrate as substrate by Lineweaver-Bu... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50004000 ((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Time dependent inhibition of equine serum BChE assessed as stability constants of inhibitor-enzyme complex using acetylthiocholine as substrate after... | ACS Med Chem Lett 3: 914-919 (2012) Article DOI: 10.1021/ml3001825 BindingDB Entry DOI: 10.7270/Q2P27079 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50000766 (CHEMBL12 | DIAZEPAM | US9271961, Diazepam) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Equus caballus (horse) serum butyrylcholinesterase (BChE) assessed as inhibition of BTCh hydrolysis by Ellman method | Citation and Details Article DOI: 10.1007/s00044-005-0140-0 BindingDB Entry DOI: 10.7270/Q2CJ8HD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50000766 (CHEMBL12 | DIAZEPAM | US9271961, Diazepam) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Equus caballus (horse) serum butyrylcholinesterase (BChE) assessed as inhibition of BTCh hydrolysis by Ellman method | Citation and Details Article DOI: 10.1007/s00044-005-0140-0 BindingDB Entry DOI: 10.7270/Q2CJ8HD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 5500 total ) | Next | Last >> |