

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Isoleucine--tRNA ligase (Staphylococcus aureus) | BDBM50471296 (CHEMBL87935) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Ltd Curated by ChEMBL | Assay Description Inhibition concentration against Isoleucyl-tRNA synthetase from Staphylococcus aureus NCTC 6571 | J Med Chem 40: 2563-70 (1997) Article DOI: 10.1021/jm960738k BindingDB Entry DOI: 10.7270/Q2GH9MPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucine--tRNA ligase (Staphylococcus aureus) | BDBM50290686 (9-((E)-4-((2S,3R,4R,5S)-3,4-dihydroxy-5-(((2S,3S)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents | DrugBank PDB Article PubMed | n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Ltd Curated by ChEMBL | Assay Description Inhibition concentration against Isoleucyl-tRNA synthetase from Staphylococcus aureus NCTC 6571 | J Med Chem 40: 2563-70 (1997) Article DOI: 10.1021/jm960738k BindingDB Entry DOI: 10.7270/Q2GH9MPZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Isoleucine--tRNA ligase (Staphylococcus aureus) | BDBM50471292 (CHEMBL315230) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Ltd Curated by ChEMBL | Assay Description Inhibition concentration against Isoleucyl-tRNA synthetase from Staphylococcus aureus NCTC 6571 | J Med Chem 40: 2563-70 (1997) Article DOI: 10.1021/jm960738k BindingDB Entry DOI: 10.7270/Q2GH9MPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucine--tRNA ligase (Staphylococcus aureus) | BDBM50471294 (CHEMBL276855) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Ltd Curated by ChEMBL | Assay Description Inhibition concentration against Isoleucyl-tRNA synthetase from Staphylococcus aureus NCTC 6571 | J Med Chem 40: 2563-70 (1997) Article DOI: 10.1021/jm960738k BindingDB Entry DOI: 10.7270/Q2GH9MPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucine--tRNA ligase (Staphylococcus aureus) | BDBM50471297 (CHEMBL424608) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Ltd Curated by ChEMBL | Assay Description Inhibition concentration against Isoleucyl-tRNA synthetase from Staphylococcus aureus NCTC 6571 | J Med Chem 40: 2563-70 (1997) Article DOI: 10.1021/jm960738k BindingDB Entry DOI: 10.7270/Q2GH9MPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucine--tRNA ligase (Staphylococcus aureus) | BDBM50471293 (CHEMBL83449) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Ltd Curated by ChEMBL | Assay Description Inhibition concentration against Isoleucyl-tRNA synthetase from Staphylococcus aureus NCTC 6571 | J Med Chem 40: 2563-70 (1997) Article DOI: 10.1021/jm960738k BindingDB Entry DOI: 10.7270/Q2GH9MPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucine--tRNA ligase (Staphylococcus aureus) | BDBM50470911 (CHEMBL122825) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.45 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against isoleucyl-tRNA synthetase (IRS) of Staphylococcus aureus | J Med Chem 39: 3596-600 (1996) Article DOI: 10.1021/jm950882q BindingDB Entry DOI: 10.7270/Q2TX3J3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucine--tRNA ligase (Staphylococcus aureus) | BDBM50471295 (CHEMBL312853) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Ltd Curated by ChEMBL | Assay Description Inhibition concentration against Isoleucyl-tRNA synthetase from Staphylococcus aureus NCTC 6571 | J Med Chem 40: 2563-70 (1997) Article DOI: 10.1021/jm960738k BindingDB Entry DOI: 10.7270/Q2GH9MPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucine--tRNA ligase (Staphylococcus aureus) | BDBM50471291 (CHEMBL83394) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Ltd Curated by ChEMBL | Assay Description Inhibition concentration against isoleucyl tRNA synthetase from Staphylococcus aureus NCTC 6571 | J Med Chem 40: 2563-70 (1997) Article DOI: 10.1021/jm960738k BindingDB Entry DOI: 10.7270/Q2GH9MPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucine--tRNA ligase (Staphylococcus aureus) | BDBM50470914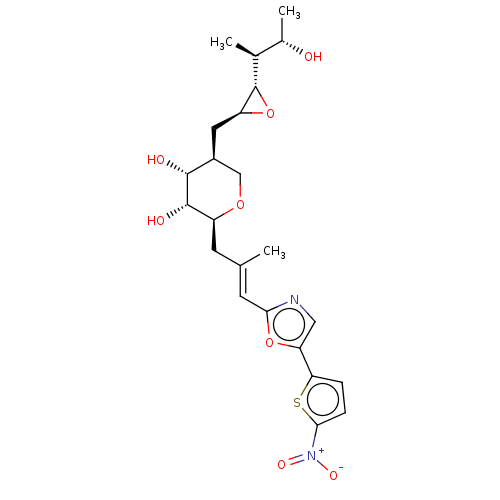 (CHEMBL120343) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.67 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against isoleucyl-tRNA synthetase (IRS) of Staphylococcus aureus NCTC 3571 | J Med Chem 39: 3596-600 (1996) Article DOI: 10.1021/jm950882q BindingDB Entry DOI: 10.7270/Q2TX3J3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucine--tRNA ligase (Staphylococcus aureus) | BDBM50470913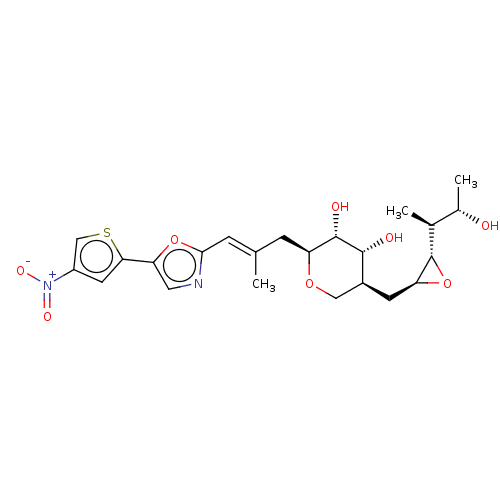 (CHEMBL120798) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.87 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against isoleucyl-tRNA synthetase (IRS) of Staphylococcus aureus | J Med Chem 39: 3596-600 (1996) Article DOI: 10.1021/jm950882q BindingDB Entry DOI: 10.7270/Q2TX3J3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucine--tRNA ligase (Staphylococcus aureus) | BDBM50470912 (CHEMBL120437) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.98 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against isoleucyl-tRNA synthetase (IRS) of Staphylococcus aureus | J Med Chem 39: 3596-600 (1996) Article DOI: 10.1021/jm950882q BindingDB Entry DOI: 10.7270/Q2TX3J3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucine--tRNA ligase (Staphylococcus aureus) | BDBM50093003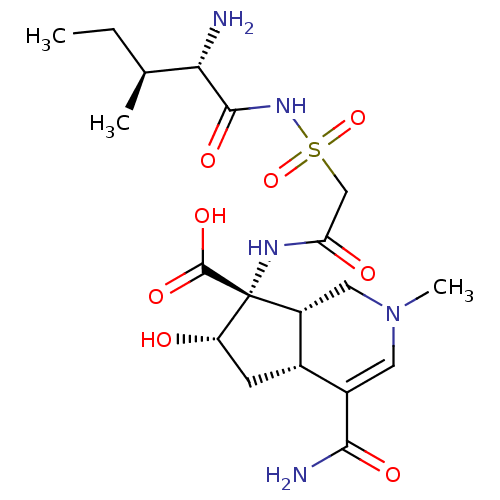 ((4aR,6S,7R,7aS)-7-[2-((2S,3S)-2-Amino-3-methyl-pen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Isoleucyl-tRNA synthetase from staphylococcus aureus WCUH29 | Bioorg Med Chem Lett 10: 2263-6 (2001) BindingDB Entry DOI: 10.7270/Q2PN94W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucine--tRNA ligase (Staphylococcus aureus) | BDBM50093001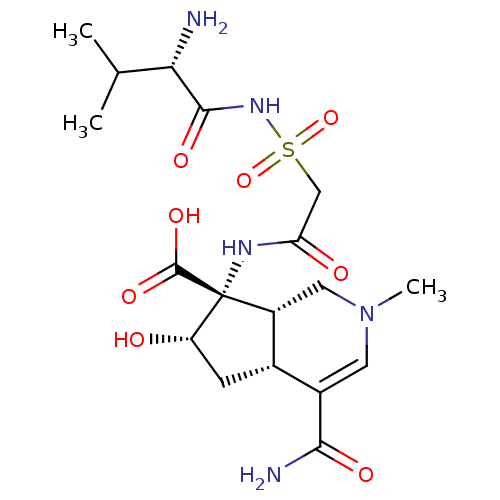 ((4aR,6S,7R,7aS)-7-[2-((S)-2-Amino-3-methyl-butyryl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Isoleucyl-tRNA synthetase from staphylococcus aureus WCUH29 | Bioorg Med Chem Lett 10: 2263-6 (2001) BindingDB Entry DOI: 10.7270/Q2PN94W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucine--tRNA ligase (Staphylococcus aureus) | BDBM50075056 (((S)-2-Amino-3-methyl-pentanoyl)-sulfamic acid (2R...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Compound tested for the inhibition of S. aureus isoleucyl-tRNA synthetase | Bioorg Med Chem Lett 9: 375-80 (1999) BindingDB Entry DOI: 10.7270/Q2M32TX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucine--tRNA ligase (Staphylococcus aureus) | BDBM50075058 (((S)-2-Amino-3-methyl-pentanoyl)-sulfamic acid (2R...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Compound tested for the inhibition of S. aureus isoleucyl-tRNA synthetase | Bioorg Med Chem Lett 9: 375-80 (1999) BindingDB Entry DOI: 10.7270/Q2M32TX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucine--tRNA ligase (Staphylococcus aureus) | BDBM50075067 (((S)-2-Amino-4-methyl-pentanoyl)-sulfamic acid (2R...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >54 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Compound tested for the inhibition of S. aureus Leucyl-tRNA synthetase | Bioorg Med Chem Lett 9: 375-80 (1999) BindingDB Entry DOI: 10.7270/Q2M32TX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucine--tRNA ligase (Staphylococcus aureus) | BDBM50093004 ((4aR,6S,7R,7aS)-7-[2-((2S,3S)-2-Amino-3-methyl-pen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Isoleucyl-tRNA synthetase from staphylococcus aureus WCUH29 | Bioorg Med Chem Lett 10: 2263-6 (2001) BindingDB Entry DOI: 10.7270/Q2PN94W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucine--tRNA ligase (Staphylococcus aureus) | BDBM50075069 (((S)-2-Amino-4-methyl-pentanoyl)-sulfamic acid (2R...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Compound tested for the inhibition of S. aureus Leucyl-tRNA synthetase | Bioorg Med Chem Lett 9: 375-80 (1999) BindingDB Entry DOI: 10.7270/Q2M32TX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucine--tRNA ligase (Staphylococcus aureus) | BDBM50075063 (((S)-2-Amino-3-methyl-pentanoyl)-sulfamic acid (2R...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Compound tested for the inhibition of S. aureus isoleucyl-tRNA synthetase | Bioorg Med Chem Lett 9: 375-80 (1999) BindingDB Entry DOI: 10.7270/Q2M32TX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucine--tRNA ligase (Staphylococcus aureus) | BDBM50075055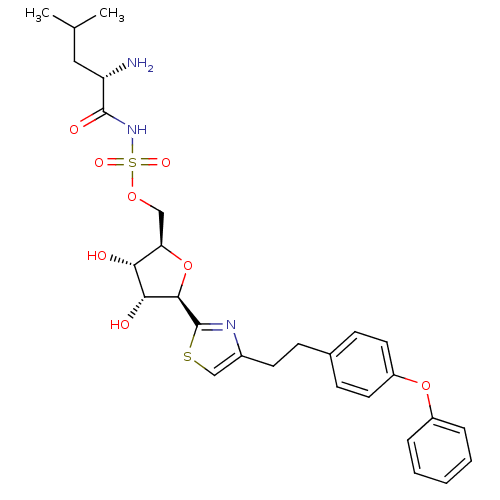 (((S)-2-Amino-4-methyl-pentanoyl)-sulfamic acid (2R...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Compound tested for the inhibition of S. aureus Leucyl-tRNA synthetase | Bioorg Med Chem Lett 9: 375-80 (1999) BindingDB Entry DOI: 10.7270/Q2M32TX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucine--tRNA ligase (Staphylococcus aureus) | BDBM50075065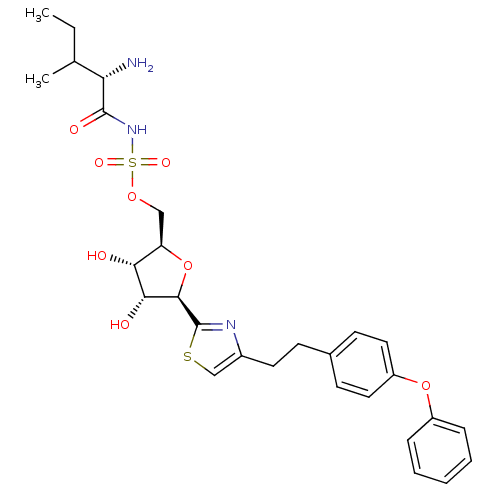 (((S)-2-Amino-3-methyl-pentanoyl)-sulfamic acid (2R...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Compound tested for the inhibition of S. aureus isoleucyl-tRNA synthetase | Bioorg Med Chem Lett 9: 375-80 (1999) BindingDB Entry DOI: 10.7270/Q2M32TX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucine--tRNA ligase (Staphylococcus aureus) | BDBM50093002 ((4aR,6S,7R,7aS)-7-[2-((2S,3S)-2-Amino-3-methyl-pen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Isoleucyl-tRNA synthetase from staphylococcus aureus WCUH29 | Bioorg Med Chem Lett 10: 2263-6 (2001) BindingDB Entry DOI: 10.7270/Q2PN94W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucine--tRNA ligase (Staphylococcus aureus) | BDBM50075061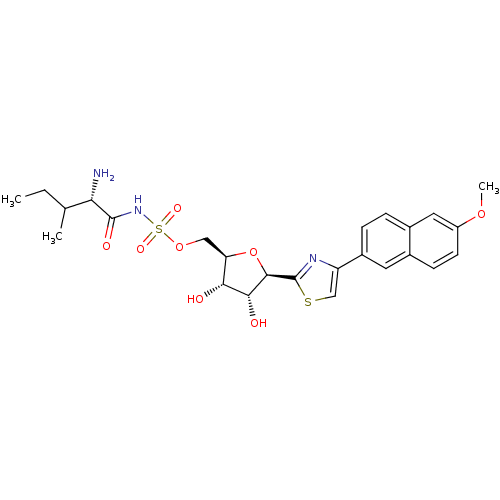 (((S)-2-Amino-3-methyl-pentanoyl)-sulfamic acid (2R...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Compound tested for the inhibition of S. aureus isoleucyl-tRNA synthetase | Bioorg Med Chem Lett 9: 375-80 (1999) BindingDB Entry DOI: 10.7270/Q2M32TX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucine--tRNA ligase (Staphylococcus aureus) | BDBM50075062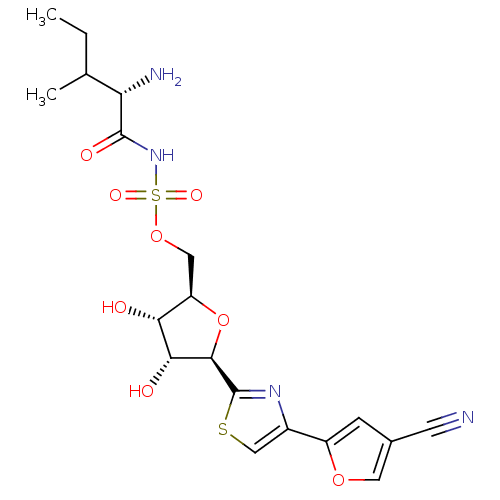 (((S)-2-Amino-3-methyl-pentanoyl)-sulfamic acid (2R...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Compound tested for the inhibition of S. aureus isoleucyl-tRNA synthetase | Bioorg Med Chem Lett 9: 375-80 (1999) BindingDB Entry DOI: 10.7270/Q2M32TX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucine--tRNA ligase (Staphylococcus aureus) | BDBM50470914 (CHEMBL120343) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 242 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against isoleucyl-tRNA synthetase (IRS) of Staphylococcus aureus 11481 | J Med Chem 39: 3596-600 (1996) Article DOI: 10.1021/jm950882q BindingDB Entry DOI: 10.7270/Q2TX3J3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucine--tRNA ligase (Staphylococcus aureus) | BDBM50075070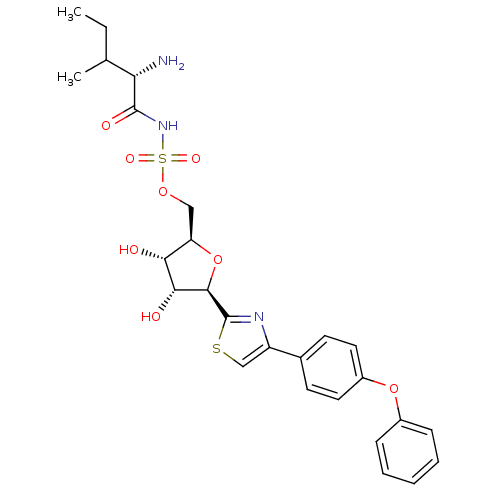 (((S)-2-Amino-3-methyl-pentanoyl)-sulfamic acid (2R...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Compound tested for the inhibition of S. aureus isoleucyl-tRNA synthetase | Bioorg Med Chem Lett 9: 375-80 (1999) BindingDB Entry DOI: 10.7270/Q2M32TX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucine--tRNA ligase (Staphylococcus aureus) | BDBM50290686 (9-((E)-4-((2S,3R,4R,5S)-3,4-dihydroxy-5-(((2S,3S)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents | DrugBank PDB Article PubMed | n/a | n/a | 279 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against isoleucyl-tRNA synthetase (IRS) of Staphylococcus aureus 11481 | J Med Chem 39: 3596-600 (1996) Article DOI: 10.1021/jm950882q BindingDB Entry DOI: 10.7270/Q2TX3J3G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Isoleucine--tRNA ligase (Staphylococcus aureus) | BDBM50075059 (((S)-2-Amino-3-methyl-pentanoyl)-sulfamic acid (2R...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Compound tested for the inhibition of S. aureus isoleucyl-tRNA synthetase | Bioorg Med Chem Lett 9: 375-80 (1999) BindingDB Entry DOI: 10.7270/Q2M32TX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucine--tRNA ligase (Staphylococcus aureus) | BDBM50093005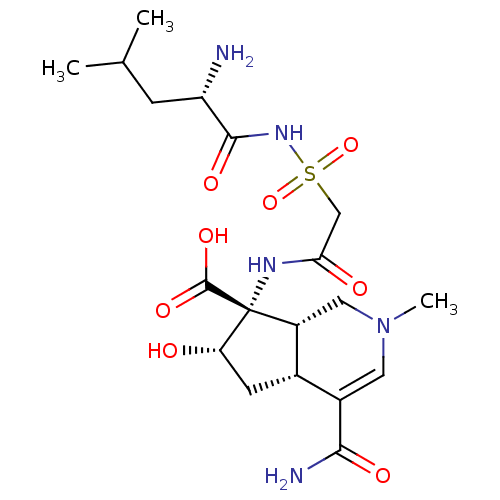 ((4aR,6S,7R,7aS)-7-[2-((S)-2-Amino-4-methyl-pentano...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Isoleucyl-tRNA synthetase from staphylococcus aureus WCUH29 | Bioorg Med Chem Lett 10: 2263-6 (2001) BindingDB Entry DOI: 10.7270/Q2PN94W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucine--tRNA ligase (Staphylococcus aureus) | BDBM50093008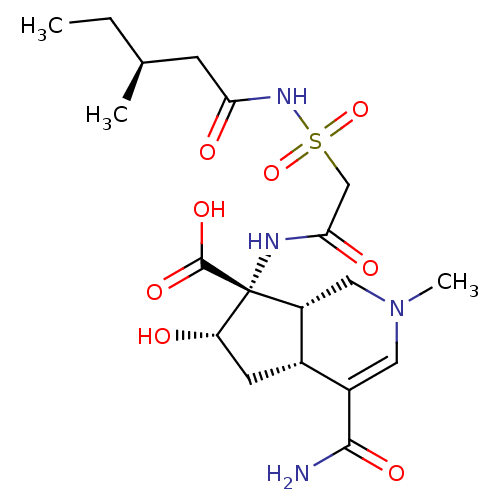 ((4aR,6S,7R,7aS)-4-Carbamoyl-6-hydroxy-2-methyl-7-[...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Isoleucyl-tRNA synthetase from staphylococcus aureus WCUH29 | Bioorg Med Chem Lett 10: 2263-6 (2001) BindingDB Entry DOI: 10.7270/Q2PN94W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucine--tRNA ligase (Staphylococcus aureus) | BDBM50075068 (((2S,3S)-2-Amino-3-methyl-pentanoyl)-sulfamic acid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Compound tested for the inhibition of S. aureus isoleucyl-tRNA synthetase | Bioorg Med Chem Lett 9: 375-80 (1999) BindingDB Entry DOI: 10.7270/Q2M32TX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucine--tRNA ligase (Staphylococcus aureus) | BDBM50093007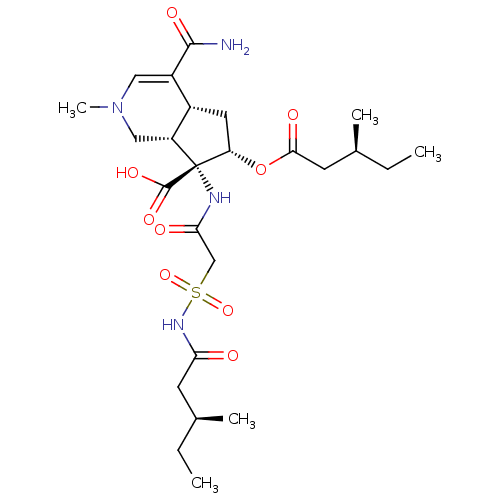 ((4aR,6S,7R,7aS)-4-Carbamoyl-2-methyl-6-((S)-3-meth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.46E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Isoleucyl-tRNA synthetase from staphylococcus aureus WCUH29 | Bioorg Med Chem Lett 10: 2263-6 (2001) BindingDB Entry DOI: 10.7270/Q2PN94W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucine--tRNA ligase (Staphylococcus aureus) | BDBM50290686 (9-((E)-4-((2S,3R,4R,5S)-3,4-dihydroxy-5-(((2S,3S)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents | DrugBank PDB Article PubMed | n/a | n/a | 3.99E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against isoleucyl-tRNA synthetase (IRS) of Staphylococcus aureus C 7 | J Med Chem 39: 3596-600 (1996) Article DOI: 10.1021/jm950882q BindingDB Entry DOI: 10.7270/Q2TX3J3G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Isoleucine--tRNA ligase (Staphylococcus aureus) | BDBM50470915 (CHEMBL118698) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.35E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description compound was tested for inhibitory activity of isoleucyl-tRNA synthetase (IRS). | J Med Chem 39: 3596-600 (1996) Article DOI: 10.1021/jm950882q BindingDB Entry DOI: 10.7270/Q2TX3J3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucine--tRNA ligase (Staphylococcus aureus) | BDBM50470915 (CHEMBL118698) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.35E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description compound was tested for inhibitory activity of isoleucyl-tRNA synthetase (IRS). | J Med Chem 39: 3596-600 (1996) Article DOI: 10.1021/jm950882q BindingDB Entry DOI: 10.7270/Q2TX3J3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucine--tRNA ligase (Staphylococcus aureus) | BDBM50470915 (CHEMBL118698) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.35E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description compound was tested for inhibitory activity of isoleucyl-tRNA synthetase (IRS). | J Med Chem 39: 3596-600 (1996) Article DOI: 10.1021/jm950882q BindingDB Entry DOI: 10.7270/Q2TX3J3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||