

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Urease subunit alpha/beta/gamma (Bacillus pasteurii) | BDBM24969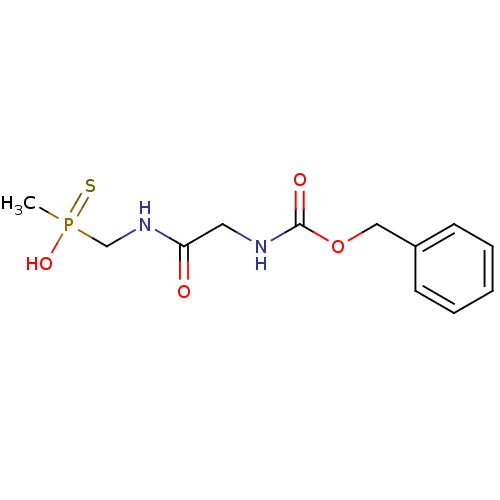 ([(2-{[(benzyloxy)carbonyl]amino}acetamido)methyl](...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 170 | -39.3 | 1.80E+3 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Wroclaw University of Technology | Assay Description Progress curves were obtained by initiation of urease reaction with addition of purified enzyme into assay mixtures containing increasing concentrati... | J Med Chem 51: 5736-44 (2008) Article DOI: 10.1021/jm800570q BindingDB Entry DOI: 10.7270/Q2PR7T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit alpha/beta/gamma (Bacillus pasteurii) | BDBM24971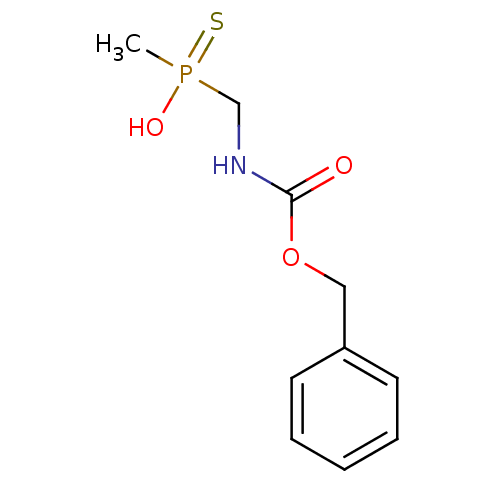 (({[(benzyloxy)carbonyl]amino}methyl)(methyl)sulfan...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.75E+4 | -27.6 | 1.12E+5 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Wroclaw University of Technology | Assay Description Progress curves were obtained by initiation of urease reaction with addition of purified enzyme into assay mixtures containing increasing concentrati... | J Med Chem 51: 5736-44 (2008) Article DOI: 10.1021/jm800570q BindingDB Entry DOI: 10.7270/Q2PR7T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit alpha/beta/gamma (Bacillus pasteurii) | BDBM24967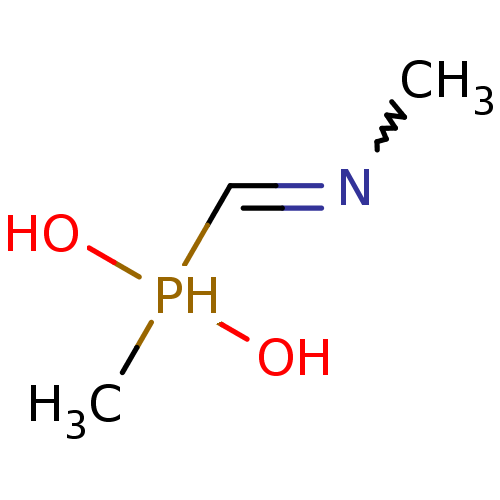 (methyl[(methylamino)methyl]phosphinic acid | organ...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.80E+4 | -27.5 | 6.00E+4 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Wroclaw University of Technology | Assay Description Progress curves were obtained by initiation of urease reaction with addition of purified enzyme into assay mixtures containing increasing concentrati... | J Med Chem 51: 5736-44 (2008) Article DOI: 10.1021/jm800570q BindingDB Entry DOI: 10.7270/Q2PR7T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit alpha/beta/gamma (Bacillus pasteurii) | BDBM24962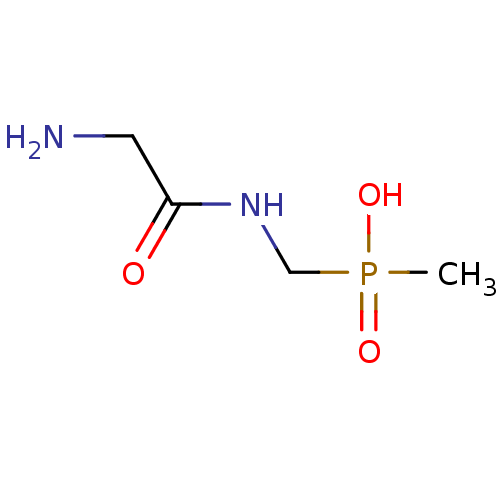 ([(2-aminoacetamido)methyl](methyl)phosphinic acid ...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.10E+4 | -27.1 | 6.00E+4 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Wroclaw University of Technology | Assay Description Progress curves were obtained by initiation of urease reaction with addition of purified enzyme into assay mixtures containing increasing concentrati... | J Med Chem 51: 5736-44 (2008) Article DOI: 10.1021/jm800570q BindingDB Entry DOI: 10.7270/Q2PR7T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit alpha/beta/gamma (Bacillus pasteurii) | BDBM24965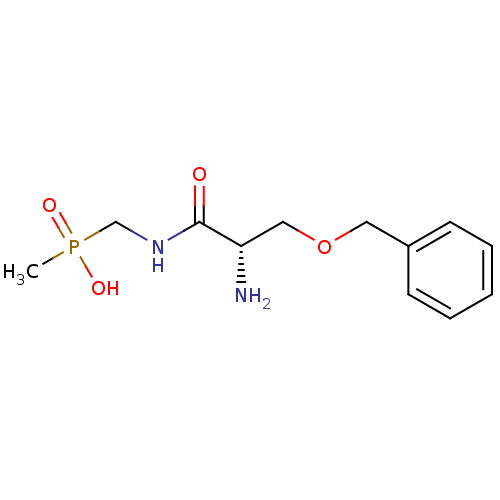 (organophosphorus derivative, 6 | {[(2S)-2-amino-3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.50E+4 | -26.7 | 8.00E+4 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Wroclaw University of Technology | Assay Description Progress curves were obtained by initiation of urease reaction with addition of purified enzyme into assay mixtures containing increasing concentrati... | J Med Chem 51: 5736-44 (2008) Article DOI: 10.1021/jm800570q BindingDB Entry DOI: 10.7270/Q2PR7T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit alpha/beta/gamma (Bacillus pasteurii) | BDBM24966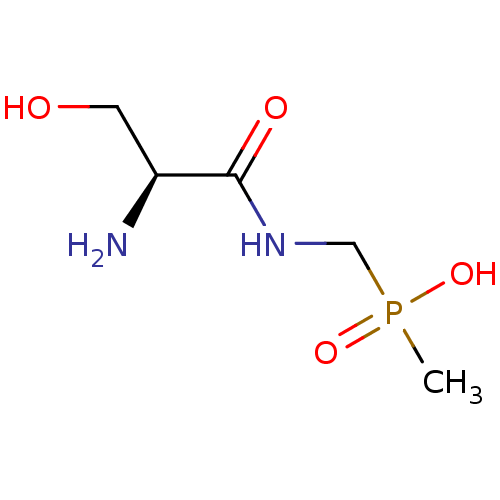 (organophosphorus derivative, 7 | {[(2S)-2-amino-3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.70E+4 | -25.7 | 1.50E+5 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Wroclaw University of Technology | Assay Description Progress curves were obtained by initiation of urease reaction with addition of purified enzyme into assay mixtures containing increasing concentrati... | J Med Chem 51: 5736-44 (2008) Article DOI: 10.1021/jm800570q BindingDB Entry DOI: 10.7270/Q2PR7T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit alpha/beta/gamma (Bacillus pasteurii) | BDBM24968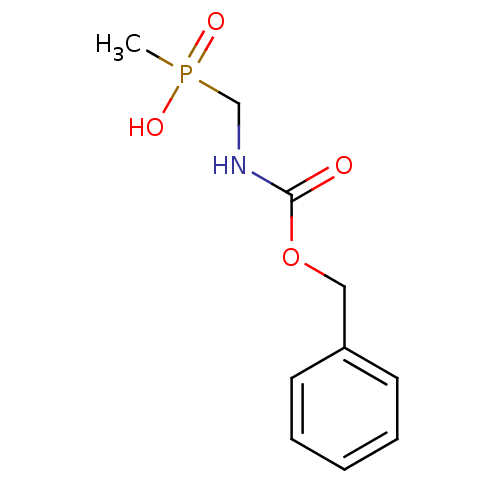 (({[(benzyloxy)carbonyl]amino}methyl)(methyl)phosph...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 4.30E+4 | -25.3 | 1.23E+5 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Wroclaw University of Technology | Assay Description Progress curves were obtained by initiation of urease reaction with addition of purified enzyme into assay mixtures containing increasing concentrati... | J Med Chem 51: 5736-44 (2008) Article DOI: 10.1021/jm800570q BindingDB Entry DOI: 10.7270/Q2PR7T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit alpha/beta/gamma (Bacillus pasteurii) | BDBM24963 (organophosphorus derivative, 4 | {[(2S)-2-amino-3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20E+5 | -22.8 | 4.85E+5 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Wroclaw University of Technology | Assay Description Progress curves were obtained by initiation of urease reaction with addition of purified enzyme into assay mixtures containing increasing concentrati... | J Med Chem 51: 5736-44 (2008) Article DOI: 10.1021/jm800570q BindingDB Entry DOI: 10.7270/Q2PR7T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit alpha/beta/gamma (Bacillus pasteurii) | BDBM24970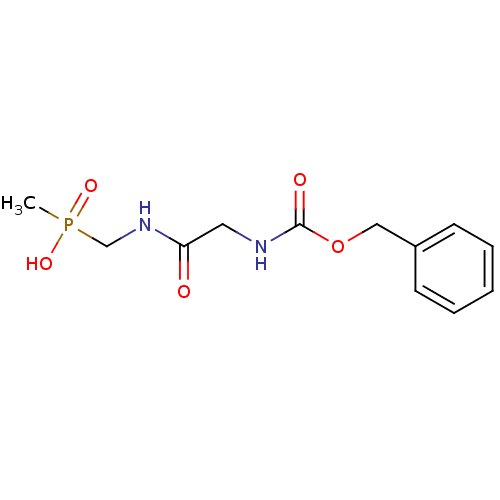 ([(2-{[(benzyloxy)carbonyl]amino}acetamido)methyl](...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.35E+5 | -22.5 | 4.50E+5 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Wroclaw University of Technology | Assay Description Progress curves were obtained by initiation of urease reaction with addition of purified enzyme into assay mixtures containing increasing concentrati... | J Med Chem 51: 5736-44 (2008) Article DOI: 10.1021/jm800570q BindingDB Entry DOI: 10.7270/Q2PR7T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit alpha/beta/gamma (Bacillus pasteurii) | BDBM24964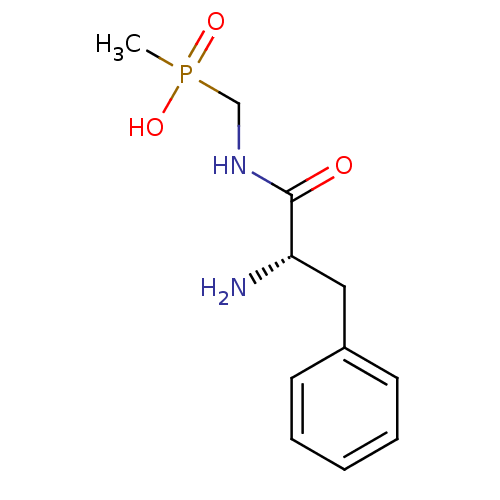 (organophosphorus derivative, 5 | {[(2S)-2-amino-3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.15E+5 | -21.3 | 6.00E+5 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Wroclaw University of Technology | Assay Description Progress curves were obtained by initiation of urease reaction with addition of purified enzyme into assay mixtures containing increasing concentrati... | J Med Chem 51: 5736-44 (2008) Article DOI: 10.1021/jm800570q BindingDB Entry DOI: 10.7270/Q2PR7T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit alpha/beta/gamma (Bacillus pasteurii) | BDBM24960 ((aminomethyl)(methyl)phosphinic acid | organophosp...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 3.40E+5 | -20.1 | 1.10E+6 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Wroclaw University of Technology | Assay Description Progress curves were obtained by initiation of urease reaction with addition of purified enzyme into assay mixtures containing increasing concentrati... | J Med Chem 51: 5736-44 (2008) Article DOI: 10.1021/jm800570q BindingDB Entry DOI: 10.7270/Q2PR7T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit alpha (Bacillus pasteurii) | BDBM173607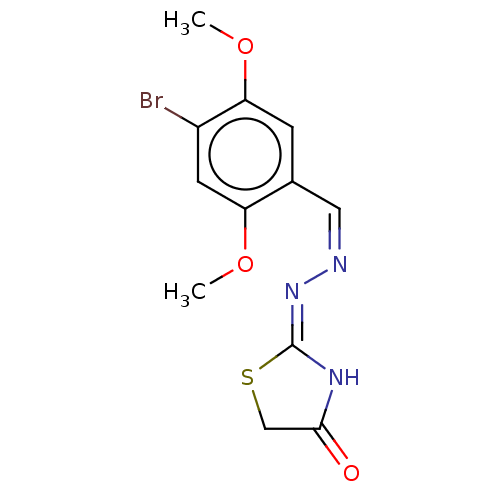 ((Z)-2-((Z)-(Napthalen-1-ylmethylene)hydrazono)thia...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.73E+3 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Hazara University | Assay Description This assay was modified from Berthelot assay and was employed for the determination of urease activity. The assay is based on the hydrolysis of urea ... | Bioorg Chem 63: 123-31 (2015) Article DOI: 10.1016/j.bioorg.2015.10.005 BindingDB Entry DOI: 10.7270/Q2H130RM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit alpha (Bacillus pasteurii) | BDBM173602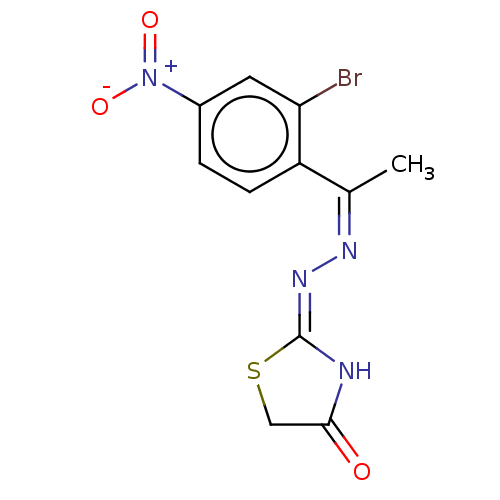 ((Z)-2-((Z)-(1-(2-Bromo-4-nitrophenyl)ethylidene)hy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.31E+3 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Hazara University | Assay Description This assay was modified from Berthelot assay and was employed for the determination of urease activity. The assay is based on the hydrolysis of urea ... | Bioorg Chem 63: 123-31 (2015) Article DOI: 10.1016/j.bioorg.2015.10.005 BindingDB Entry DOI: 10.7270/Q2H130RM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit alpha (Bacillus pasteurii) | BDBM173604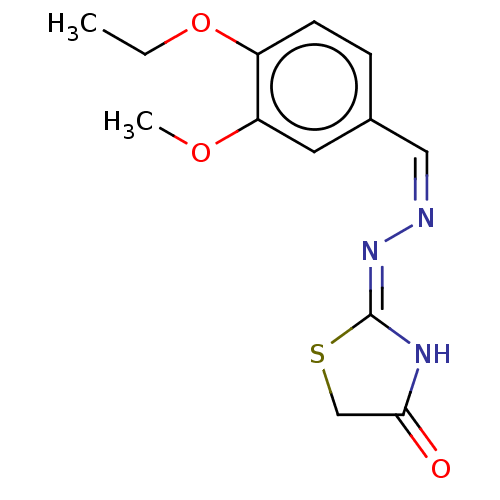 ((Z)-2-((Z)-(4-Ethoxy-3-methoxybenzoylidine)hydrazo...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.75E+3 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Hazara University | Assay Description This assay was modified from Berthelot assay and was employed for the determination of urease activity. The assay is based on the hydrolysis of urea ... | Bioorg Chem 63: 123-31 (2015) Article DOI: 10.1016/j.bioorg.2015.10.005 BindingDB Entry DOI: 10.7270/Q2H130RM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit alpha (Bacillus pasteurii) | BDBM173597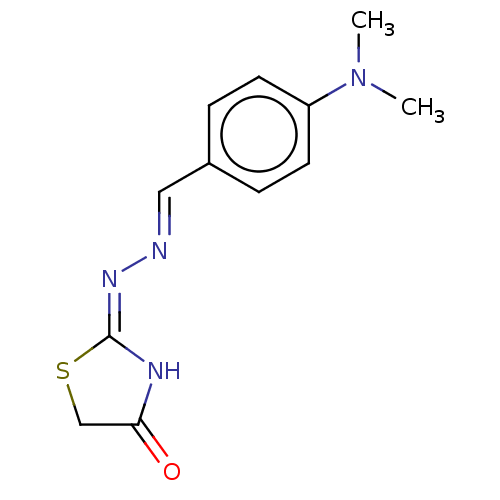 ((E)-2-((E)-(4-(Benzyloxy)-2-methoxybenzylidene)hyd...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Hazara University | Assay Description This assay was modified from Berthelot assay and was employed for the determination of urease activity. The assay is based on the hydrolysis of urea ... | Bioorg Chem 63: 123-31 (2015) Article DOI: 10.1016/j.bioorg.2015.10.005 BindingDB Entry DOI: 10.7270/Q2H130RM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit alpha (Bacillus pasteurii) | BDBM173595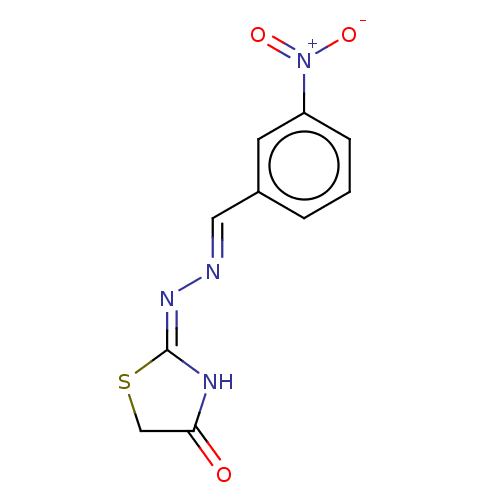 ((E)-2((E)-(3-nitrobenzylidene)hydrazono)thiazolidi...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.43E+3 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Hazara University | Assay Description This assay was modified from Berthelot assay and was employed for the determination of urease activity. The assay is based on the hydrolysis of urea ... | Bioorg Chem 63: 123-31 (2015) Article DOI: 10.1016/j.bioorg.2015.10.005 BindingDB Entry DOI: 10.7270/Q2H130RM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit alpha (Bacillus pasteurii) | BDBM60584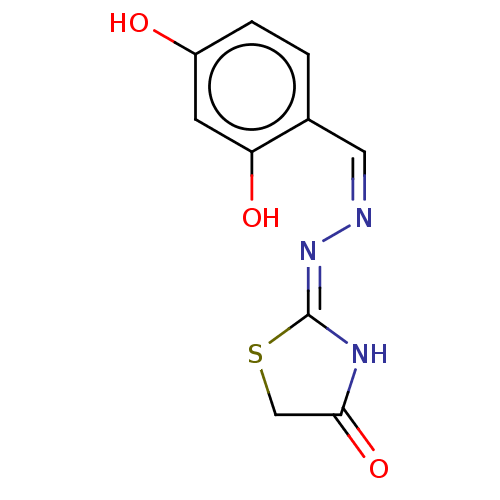 ((Z)-2-((Z)-(Napthalen-1-ylmethylene)hydrazono)thia...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.81E+3 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Hazara University | Assay Description This assay was modified from Berthelot assay and was employed for the determination of urease activity. The assay is based on the hydrolysis of urea ... | Bioorg Chem 63: 123-31 (2015) Article DOI: 10.1016/j.bioorg.2015.10.005 BindingDB Entry DOI: 10.7270/Q2H130RM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit alpha (Bacillus pasteurii) | BDBM173590 ((E)-2-((E)-(3-Chloro-4-hydroxybenzylideno)thiazoli...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.34E+3 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Hazara University | Assay Description This assay was modified from Berthelot assay and was employed for the determination of urease activity. The assay is based on the hydrolysis of urea ... | Bioorg Chem 63: 123-31 (2015) Article DOI: 10.1016/j.bioorg.2015.10.005 BindingDB Entry DOI: 10.7270/Q2H130RM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit alpha (Bacillus pasteurii) | BDBM181105 (3-(3,4,5-Trimethoxybenzoyl)-(4-hydroxycoumarin) (9...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.13E+4 | n/a | n/a | n/a | n/a | n/a | 30 |
COMSATS Institute of Information Technology | Assay Description Reaction mixtures comprising one unit of urease enzyme (B. pasteurii) solution and 55 無 of buffers containing 100 mMol urea were incubated with 5 無... | Bioorg Chem 66: 111-6 (2016) Article DOI: 10.1016/j.bioorg.2016.04.005 BindingDB Entry DOI: 10.7270/Q2QZ28RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit alpha (Bacillus pasteurii) | BDBM181103 (3-Decanoyl-(4-hydroxycoumarin) (5)) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | 30 |
COMSATS Institute of Information Technology | Assay Description Reaction mixtures comprising one unit of urease enzyme (B. pasteurii) solution and 55 無 of buffers containing 100 mMol urea were incubated with 5 無... | Bioorg Chem 66: 111-6 (2016) Article DOI: 10.1016/j.bioorg.2016.04.005 BindingDB Entry DOI: 10.7270/Q2QZ28RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit alpha (Bacillus pasteurii) | BDBM173593 ((E)-2((E)-(4-Hydroxybenzylidin)hydrazono)thiazolid...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.46E+4 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Hazara University | Assay Description This assay was modified from Berthelot assay and was employed for the determination of urease activity. The assay is based on the hydrolysis of urea ... | Bioorg Chem 63: 123-31 (2015) Article DOI: 10.1016/j.bioorg.2015.10.005 BindingDB Entry DOI: 10.7270/Q2H130RM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit alpha (Bacillus pasteurii) | BDBM181104 (3-Lauroyl-(4-hydroxycoumarin) (6)) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.71E+4 | n/a | n/a | n/a | n/a | n/a | 30 |
COMSATS Institute of Information Technology | Assay Description Reaction mixtures comprising one unit of urease enzyme (B. pasteurii) solution and 55 無 of buffers containing 100 mMol urea were incubated with 5 無... | Bioorg Chem 66: 111-6 (2016) Article DOI: 10.1016/j.bioorg.2016.04.005 BindingDB Entry DOI: 10.7270/Q2QZ28RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit alpha (Bacillus pasteurii) | BDBM50229993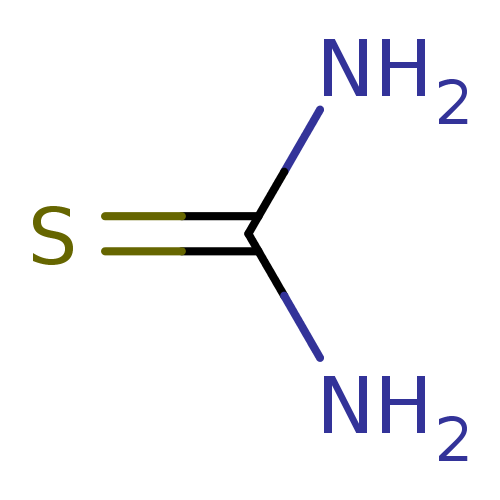 (2-thiourea | CHEMBL260876 | Thiocarbamid | Thiohar...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | 30 |
COMSATS Institute of Information Technology | Assay Description Reaction mixtures comprising one unit of urease enzyme (B. pasteurii) solution and 55 無 of buffers containing 100 mMol urea were incubated with 5 無... | Bioorg Chem 66: 111-6 (2016) Article DOI: 10.1016/j.bioorg.2016.04.005 BindingDB Entry DOI: 10.7270/Q2QZ28RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit alpha (Bacillus pasteurii) | BDBM50229993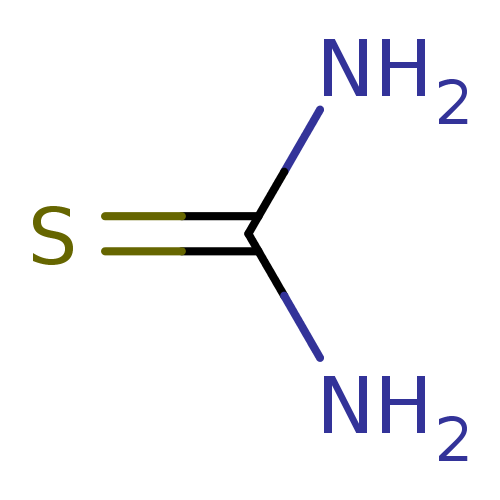 (2-thiourea | CHEMBL260876 | Thiocarbamid | Thiohar...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 2.13E+4 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Hazara University | Assay Description This assay was modified from Berthelot assay and was employed for the determination of urease activity. The assay is based on the hydrolysis of urea ... | Bioorg Chem 63: 123-31 (2015) Article DOI: 10.1016/j.bioorg.2015.10.005 BindingDB Entry DOI: 10.7270/Q2H130RM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit alpha (Bacillus pasteurii) | BDBM173592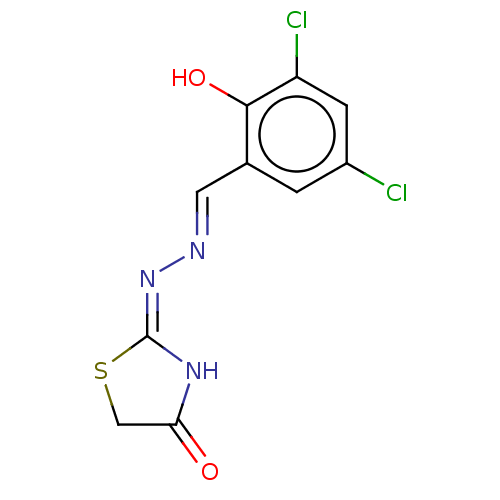 ((E)-2-((E)-(3,5-Dichloro-2-hydroxybenzylidene)hydr...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.67E+4 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Hazara University | Assay Description This assay was modified from Berthelot assay and was employed for the determination of urease activity. The assay is based on the hydrolysis of urea ... | Bioorg Chem 63: 123-31 (2015) Article DOI: 10.1016/j.bioorg.2015.10.005 BindingDB Entry DOI: 10.7270/Q2H130RM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit alpha (Bacillus pasteurii) | BDBM173589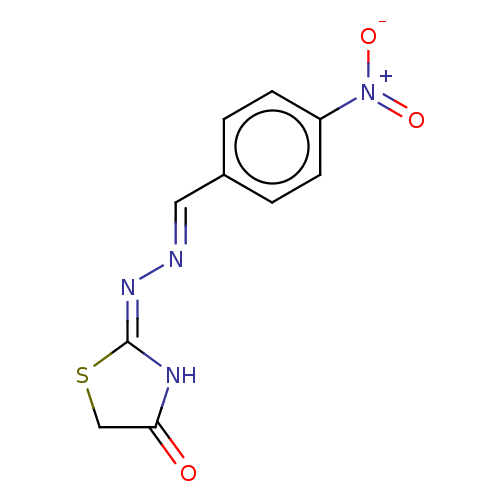 ((E)-2((E)-(4-Nitrobenzylidene)hydrazono)thiazolidi...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.69E+4 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Hazara University | Assay Description This assay was modified from Berthelot assay and was employed for the determination of urease activity. The assay is based on the hydrolysis of urea ... | Bioorg Chem 63: 123-31 (2015) Article DOI: 10.1016/j.bioorg.2015.10.005 BindingDB Entry DOI: 10.7270/Q2H130RM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit alpha (Bacillus pasteurii) | BDBM181106 (3-(Methanesulfonoyl)-(4-hydroxycoumarin) (11)) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.95E+4 | n/a | n/a | n/a | n/a | n/a | 30 |
COMSATS Institute of Information Technology | Assay Description Reaction mixtures comprising one unit of urease enzyme (B. pasteurii) solution and 55 無 of buffers containing 100 mMol urea were incubated with 5 無... | Bioorg Chem 66: 111-6 (2016) Article DOI: 10.1016/j.bioorg.2016.04.005 BindingDB Entry DOI: 10.7270/Q2QZ28RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit alpha (Bacillus pasteurii) | BDBM181102 (3-Nonanoyl-(4-hydroxycoumarin) (4)) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.09E+4 | n/a | n/a | n/a | n/a | n/a | 30 |
COMSATS Institute of Information Technology | Assay Description Reaction mixtures comprising one unit of urease enzyme (B. pasteurii) solution and 55 無 of buffers containing 100 mMol urea were incubated with 5 無... | Bioorg Chem 66: 111-6 (2016) Article DOI: 10.1016/j.bioorg.2016.04.005 BindingDB Entry DOI: 10.7270/Q2QZ28RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit alpha (Bacillus pasteurii) | BDBM173594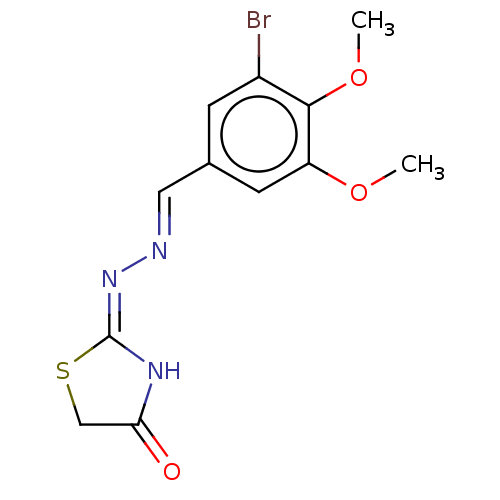 ((E)-2((E)-(3-Bromo-4,5-dimethoxybenzylidene)hydraz...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.66E+4 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Hazara University | Assay Description This assay was modified from Berthelot assay and was employed for the determination of urease activity. The assay is based on the hydrolysis of urea ... | Bioorg Chem 63: 123-31 (2015) Article DOI: 10.1016/j.bioorg.2015.10.005 BindingDB Entry DOI: 10.7270/Q2H130RM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit alpha (Bacillus pasteurii) | BDBM173596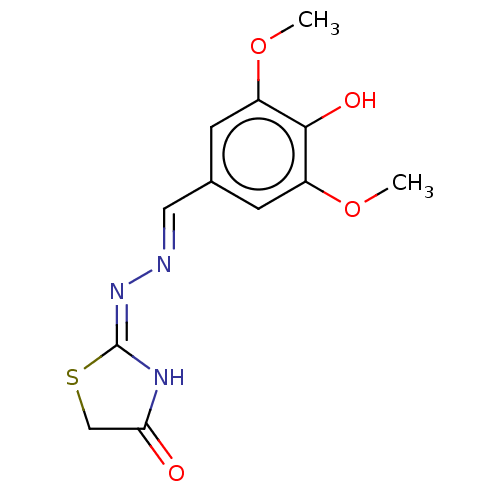 ((E)-2-((E)-(4-Hyrazoxy-3,5-dimethoxybenzylidene)hy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.79E+4 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Hazara University | Assay Description This assay was modified from Berthelot assay and was employed for the determination of urease activity. The assay is based on the hydrolysis of urea ... | Bioorg Chem 63: 123-31 (2015) Article DOI: 10.1016/j.bioorg.2015.10.005 BindingDB Entry DOI: 10.7270/Q2H130RM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit alpha (Bacillus pasteurii) | BDBM173600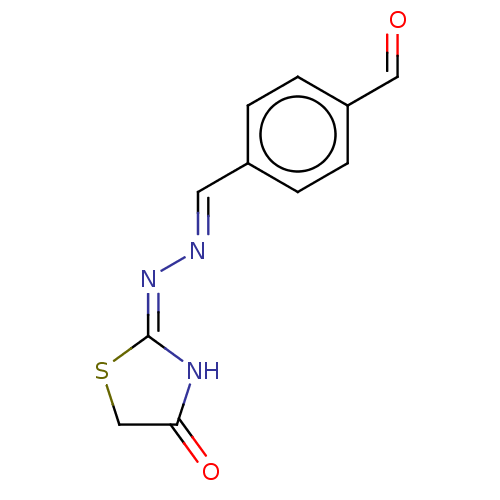 ((E)-2-((E)-(4-(Benzyloxy)benzylidene)hydrazono)thi...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.92E+4 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Hazara University | Assay Description This assay was modified from Berthelot assay and was employed for the determination of urease activity. The assay is based on the hydrolysis of urea ... | Bioorg Chem 63: 123-31 (2015) Article DOI: 10.1016/j.bioorg.2015.10.005 BindingDB Entry DOI: 10.7270/Q2H130RM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit alpha (Bacillus pasteurii) | BDBM173601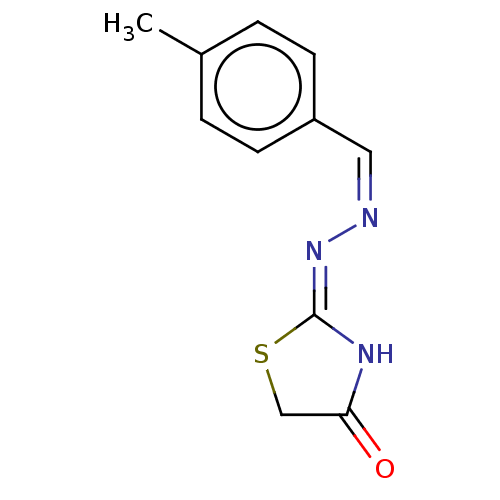 ((2Z)-2,20(Z)-(1,4-Phenylenebis(methane-1-yl-yliden...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.39E+4 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Hazara University | Assay Description This assay was modified from Berthelot assay and was employed for the determination of urease activity. The assay is based on the hydrolysis of urea ... | Bioorg Chem 63: 123-31 (2015) Article DOI: 10.1016/j.bioorg.2015.10.005 BindingDB Entry DOI: 10.7270/Q2H130RM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit alpha (Bacillus pasteurii) | BDBM173588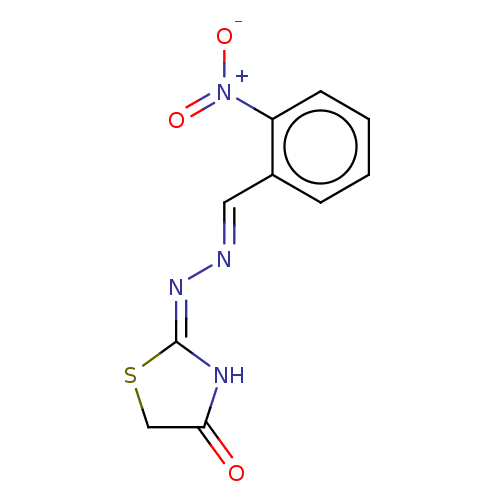 ((E)-2-((E)-(2-Nitrobenzylidene)hydrazono)thiazolid...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.75E+4 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Hazara University | Assay Description This assay was modified from Berthelot assay and was employed for the determination of urease activity. The assay is based on the hydrolysis of urea ... | Bioorg Chem 63: 123-31 (2015) Article DOI: 10.1016/j.bioorg.2015.10.005 BindingDB Entry DOI: 10.7270/Q2H130RM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit alpha (Bacillus pasteurii) | BDBM173598 (4-((E)-((E)-(4-Oxothiazolidin-2-ylidene)hydrazono)...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.85E+4 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Hazara University | Assay Description This assay was modified from Berthelot assay and was employed for the determination of urease activity. The assay is based on the hydrolysis of urea ... | Bioorg Chem 63: 123-31 (2015) Article DOI: 10.1016/j.bioorg.2015.10.005 BindingDB Entry DOI: 10.7270/Q2H130RM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit alpha (Bacillus pasteurii) | BDBM173603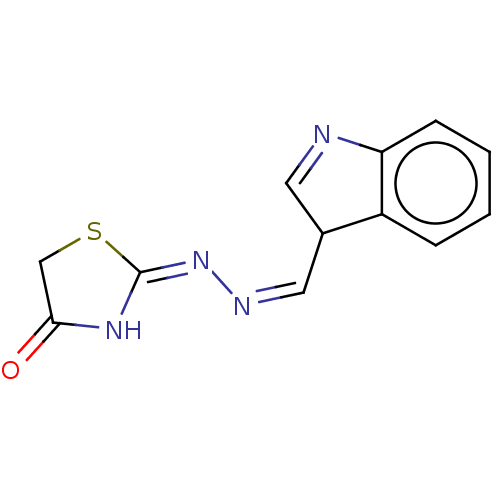 ((E)-2-(E)-((1H-Indole-3-yl)methylene)hydrazono)thi...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.14E+4 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Hazara University | Assay Description This assay was modified from Berthelot assay and was employed for the determination of urease activity. The assay is based on the hydrolysis of urea ... | Bioorg Chem 63: 123-31 (2015) Article DOI: 10.1016/j.bioorg.2015.10.005 BindingDB Entry DOI: 10.7270/Q2H130RM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit alpha (Bacillus pasteurii) | BDBM173591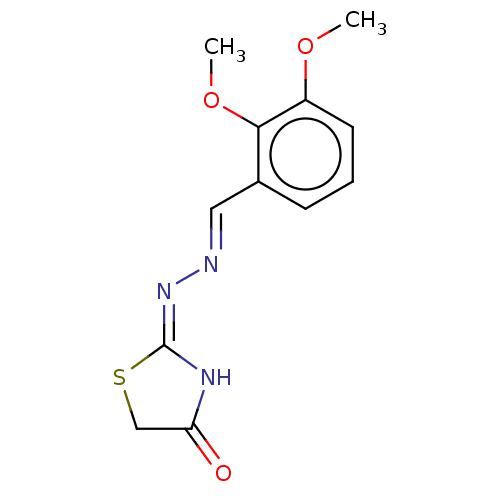 ((E)-2-((E)-(2,3-Dimethoxybenzylidene)hydrazono)thi...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.25E+4 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Hazara University | Assay Description This assay was modified from Berthelot assay and was employed for the determination of urease activity. The assay is based on the hydrolysis of urea ... | Bioorg Chem 63: 123-31 (2015) Article DOI: 10.1016/j.bioorg.2015.10.005 BindingDB Entry DOI: 10.7270/Q2H130RM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit alpha (Bacillus pasteurii) | BDBM181107 (3-(3-Nitrobenzosulfonyl)-(4-hydroxycoumarin) (14)) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.62E+4 | n/a | n/a | n/a | n/a | n/a | 30 |
COMSATS Institute of Information Technology | Assay Description Reaction mixtures comprising one unit of urease enzyme (B. pasteurii) solution and 55 無 of buffers containing 100 mMol urea were incubated with 5 無... | Bioorg Chem 66: 111-6 (2016) Article DOI: 10.1016/j.bioorg.2016.04.005 BindingDB Entry DOI: 10.7270/Q2QZ28RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit alpha (Bacillus pasteurii) | BDBM173599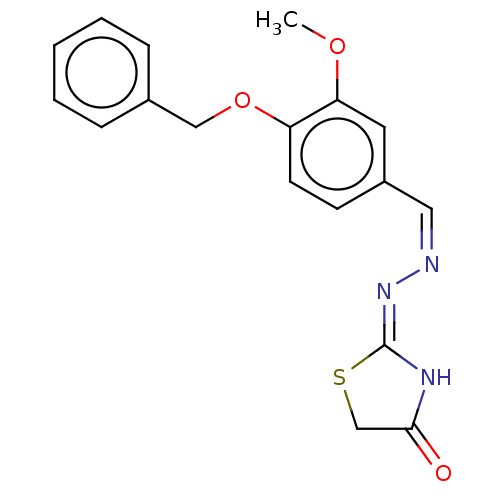 ((Z)-2-((Z)-(4-(Dimethyl amino)benzylodine)hydrazon...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.89E+4 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Hazara University | Assay Description This assay was modified from Berthelot assay and was employed for the determination of urease activity. The assay is based on the hydrolysis of urea ... | Bioorg Chem 63: 123-31 (2015) Article DOI: 10.1016/j.bioorg.2015.10.005 BindingDB Entry DOI: 10.7270/Q2H130RM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit alpha (Bacillus pasteurii) | BDBM181108 (3-(1-Naphthosulfonyl)-(4-hydroxycoumarin) (16)) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.95E+4 | n/a | n/a | n/a | n/a | n/a | 30 |
COMSATS Institute of Information Technology | Assay Description Reaction mixtures comprising one unit of urease enzyme (B. pasteurii) solution and 55 無 of buffers containing 100 mMol urea were incubated with 5 無... | Bioorg Chem 66: 111-6 (2016) Article DOI: 10.1016/j.bioorg.2016.04.005 BindingDB Entry DOI: 10.7270/Q2QZ28RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit alpha (Bacillus pasteurii) | BDBM173605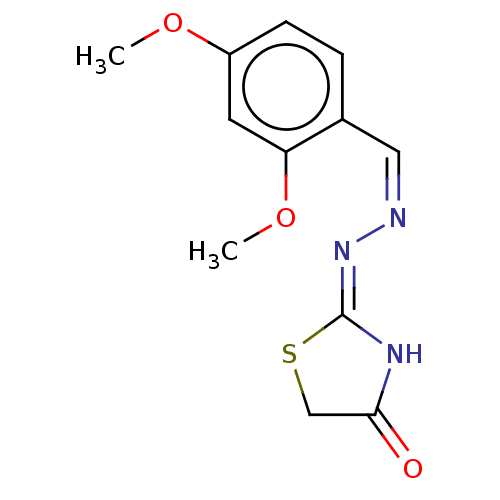 ((Z)-2-((Z)-(2,4-Dimethoxybenzoylidine)hydrazono)th...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.97E+4 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Hazara University | Assay Description This assay was modified from Berthelot assay and was employed for the determination of urease activity. The assay is based on the hydrolysis of urea ... | Bioorg Chem 63: 123-31 (2015) Article DOI: 10.1016/j.bioorg.2015.10.005 BindingDB Entry DOI: 10.7270/Q2H130RM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||