

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase transforming protein Abl (Abelson murine leukemia virus) | BDBM3032 (CHEMBL1204168 | CHEMBL29197 | N-(3-bromophenyl)-6,...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase transforming protein Abl (Abelson murine leukemia virus) | BDBM3012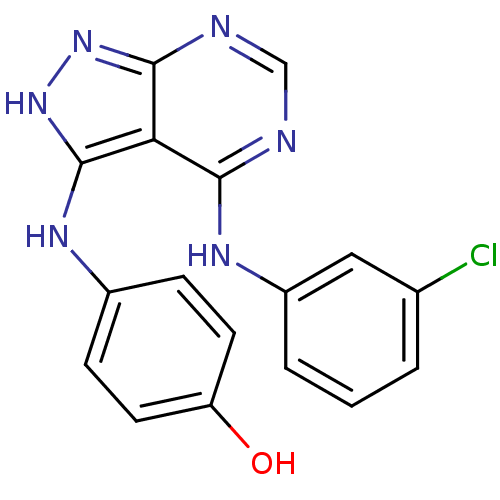 (3-((4-Hydroxyphenyl)amino)-4-((3-chlorophenyl)amin...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase transforming protein Abl (Abelson murine leukemia virus) | BDBM3023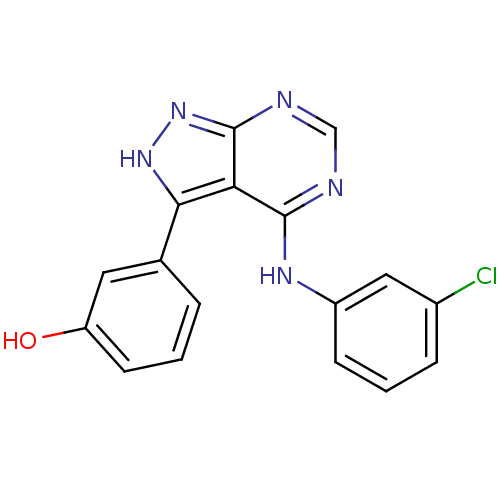 (3-{4-[(3-chlorophenyl)amino]-1H-pyrazolo[3,4-d]pyr...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase transforming protein Abl (Abelson murine leukemia virus) | BDBM3014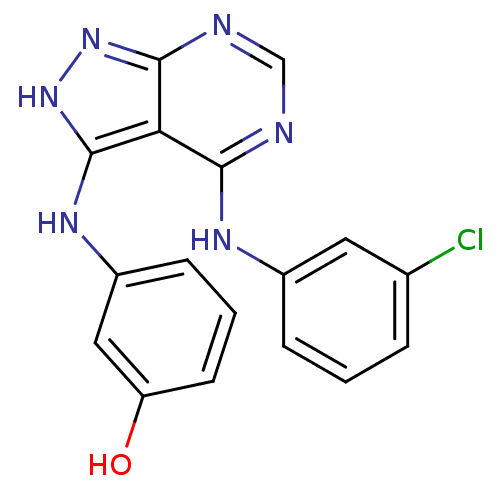 (3-((3-Hydroxyphenyl)amino)-4-((3-chlorophenyl)amin...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase transforming protein Abl (Abelson murine leukemia virus) | BDBM3005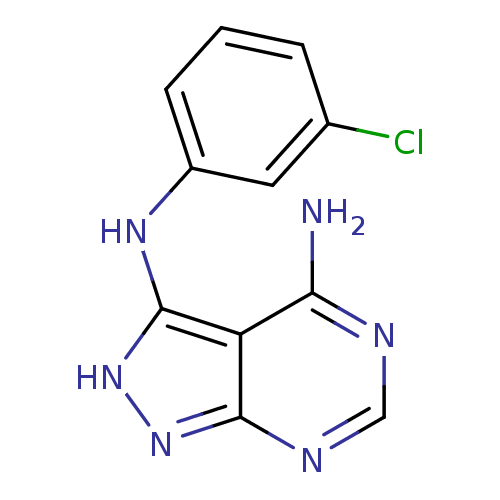 (3-((3-Chlorophenyl)amino)-4-amino-1H-pyrazolo[3,4-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | 7.5 | 20 |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase transforming protein Abl (Abelson murine leukemia virus) | BDBM3016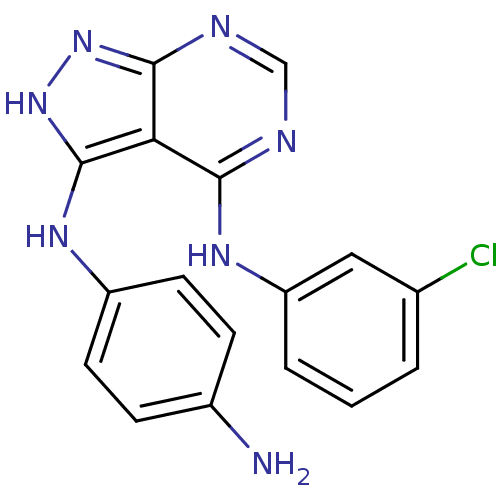 (3-((4-Aminophenyl)amino)-4-((3-chlorophenyl)amino)...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase transforming protein Abl (Abelson murine leukemia virus) | BDBM3007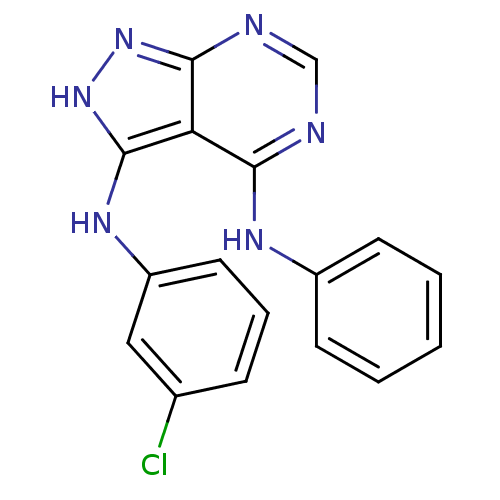 (3-((3-Chlorophenyl)amino)-4-(phenylamino)-1H-pyraz...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.36E+3 | n/a | n/a | n/a | n/a | 7.5 | 20 |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase transforming protein Abl (Abelson murine leukemia virus) | BDBM3009 (3-((3-Chlorophenyl)amino)-4-((3-bromophenyl)amino)...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase transforming protein Abl (Abelson murine leukemia virus) | BDBM3010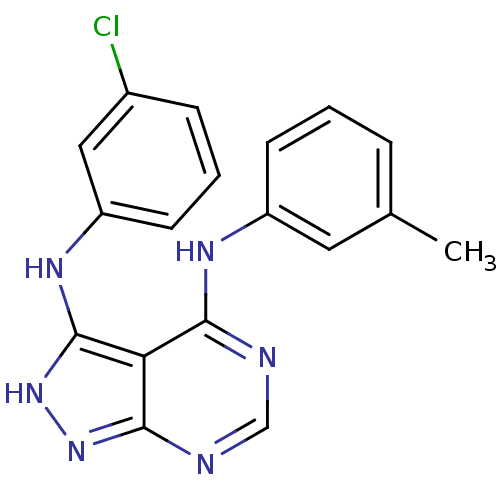 (3-((3-Chlorophenyl)amino)-4-((3-methylphenyl)amino...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase transforming protein Abl (Abelson murine leukemia virus) | BDBM3004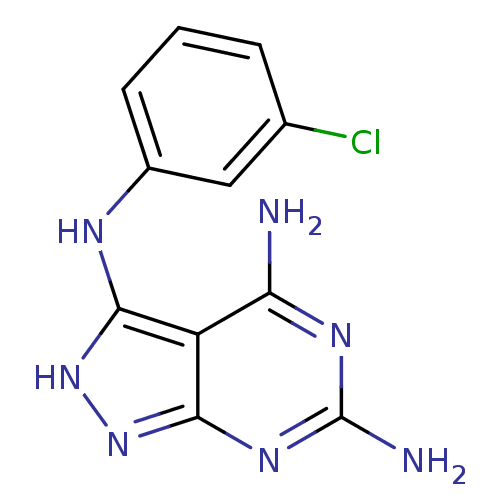 (3-((3-Chlorophenyl)amino)-4,6-diamino-1H-pyrazolo[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | 7.5 | 20 |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase transforming protein Abl (Abelson murine leukemia virus) | BDBM3006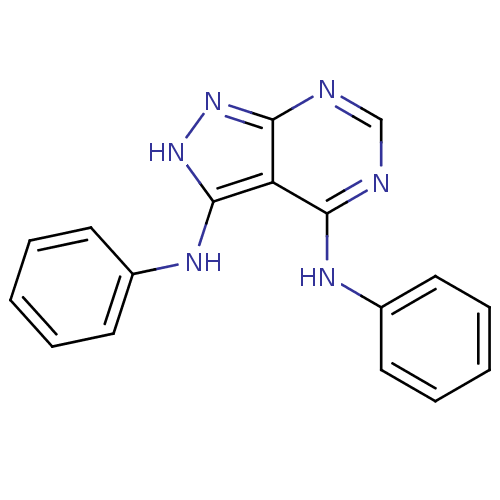 (3,4-Di-(phenylamino)-1H-pyrazolo[3,4-d]pyrimidine ...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.96E+3 | n/a | n/a | n/a | n/a | 7.5 | 20 |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase transforming protein Abl (Abelson murine leukemia virus) | BDBM3008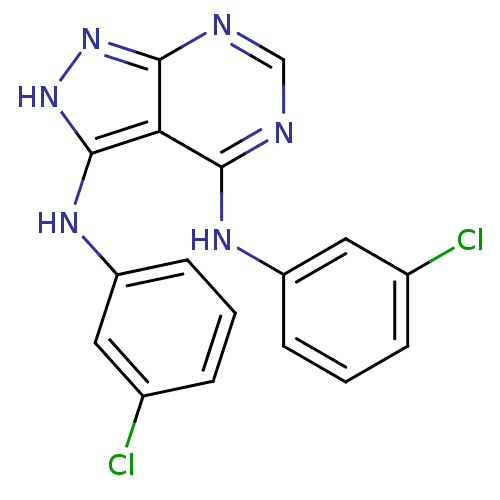 (3,4-Bis((3-chlorophenyl)amino)-1H-pyrazolo[3,4-d]p...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.98E+3 | n/a | n/a | n/a | n/a | 7.5 | 20 |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase transforming protein Abl (Abelson murine leukemia virus) | BDBM3025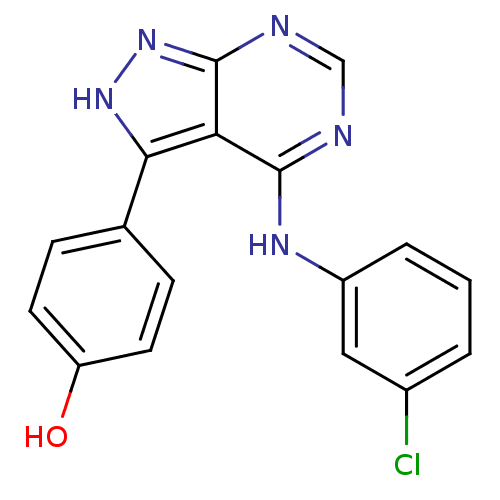 (3-(4-Hydroxyphenyl)-4-((3-chlorophenyl)amino)pyraz...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase transforming protein Abl (Abelson murine leukemia virus) | BDBM3027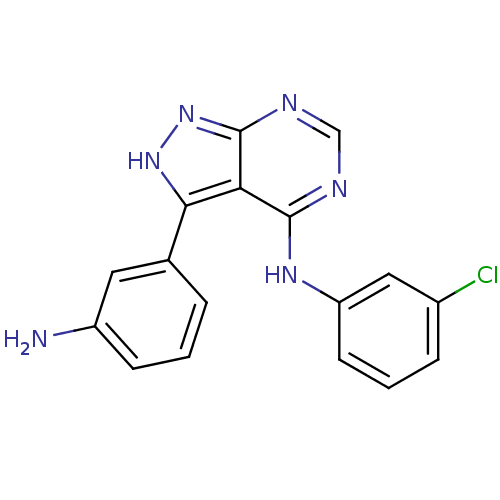 (3-(3-Aminophenyl)-4-((3-chlorophenyl)amino)-1H-pyr...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase transforming protein Abl (Abelson murine leukemia virus) | BDBM3013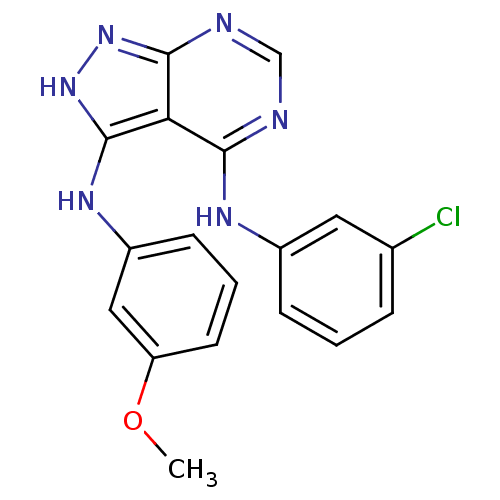 (3-((3-Methoxyphenyl)amino)-4-((3-chlorophenyl)amin...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase transforming protein Abl (Abelson murine leukemia virus) | BDBM3020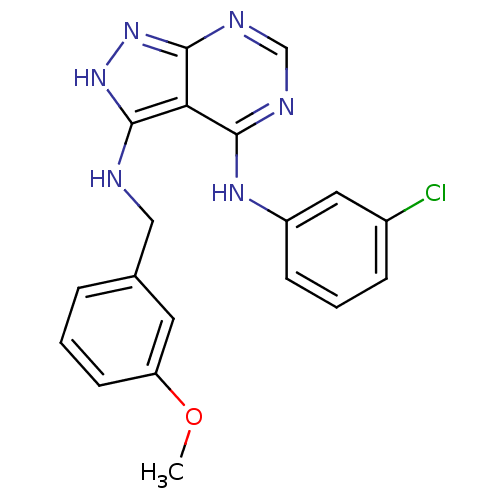 (3-((3-Methoxybenzyl)amino)-4-((3-chlorophenyl)amin...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase transforming protein Abl (Abelson murine leukemia virus) | BDBM3029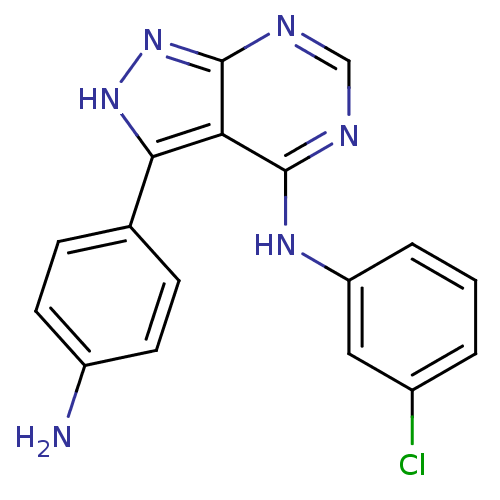 (3-(4-Aminophenyl)-4-((3-chlorophenyl)amino)-1H-pyr...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase transforming protein Abl (Abelson murine leukemia virus) | BDBM3022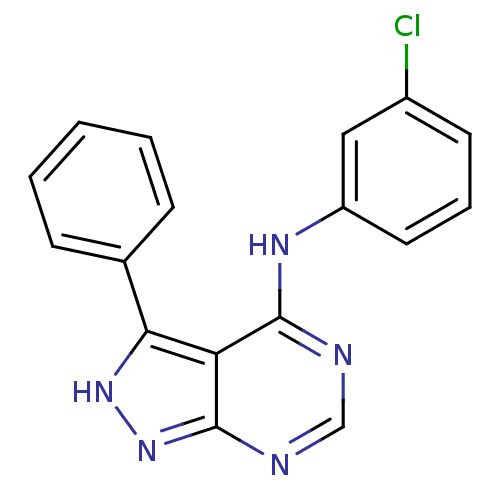 (4-(Phenylamino)pyrazolo[3,4-d]pyrimidine deriv. 23...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase transforming protein Abl (Abelson murine leukemia virus) | BDBM50292488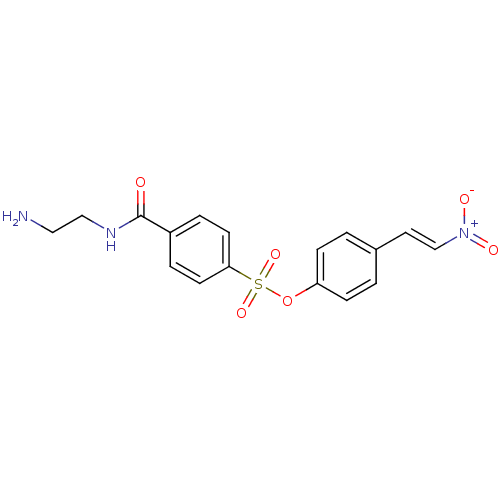 (4-(2-nitrovinyl)phenyl 4-(2-aminoethylcarbamoyl)be...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Abelson murine leukemia virus p60 v-abl | J Nat Prod 55: 1529-1560 (1992) Article DOI: 10.1021/np50089a001 BindingDB Entry DOI: 10.7270/Q2J966CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase transforming protein Abl (Abelson murine leukemia virus) | BDBM3018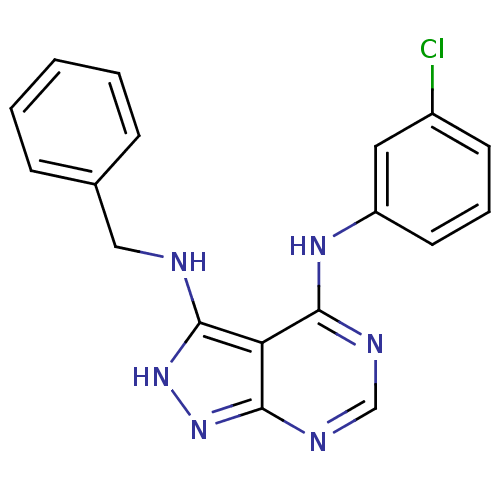 (3-(Benzylamino)-4-((3-chlorophenyl)amino)-1H-pyraz...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase transforming protein Abl (Abelson murine leukemia virus) | BDBM3031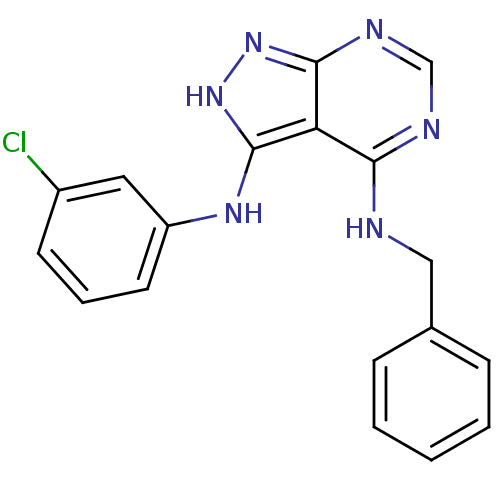 (3-((3-Chlorophenyl)amino)-4-(benzylamino)-1H-pyraz...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase transforming protein Abl (Abelson murine leukemia virus) | BDBM3030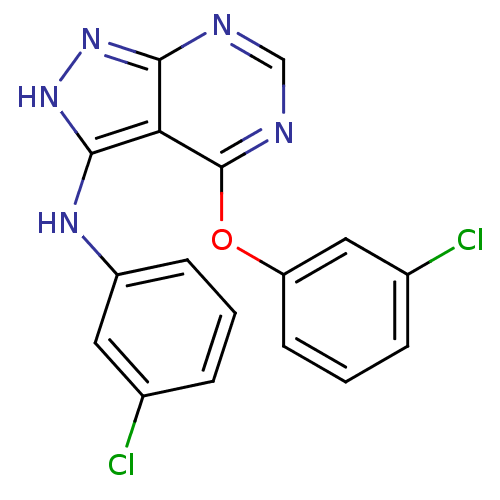 (4-(3-chlorophenoxy)-N-(3-chlorophenyl)-1H-pyrazolo...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase transforming protein Abl (Abelson murine leukemia virus) | BDBM3028 (3-(4-(BOC-amino)phenyl)-4-((3-chlorophenyl)amino)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase transforming protein Abl (Abelson murine leukemia virus) | BDBM3021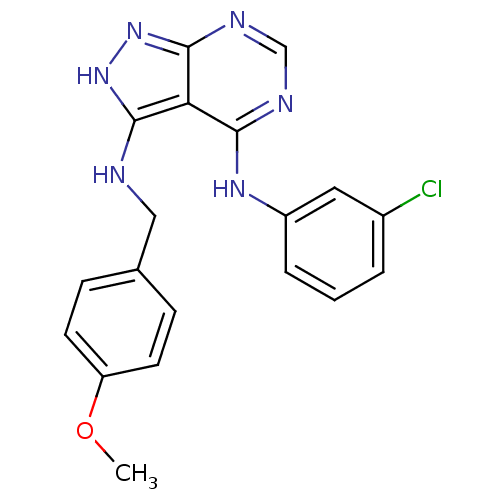 (3-((4-Methoxybenzyl)amino)-4-((3-chlorophenyl)amin...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase transforming protein Abl (Abelson murine leukemia virus) | BDBM3019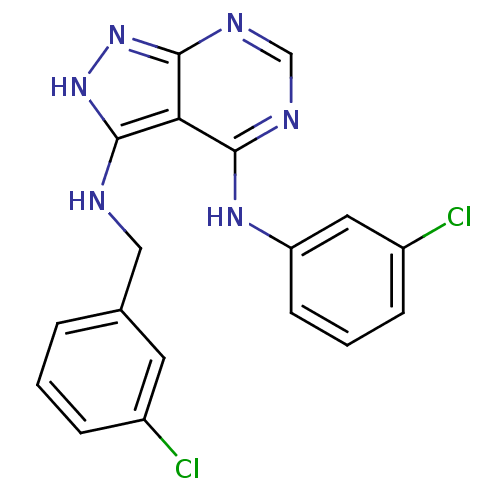 (3-((3-Chlorobenzyl)amino)-4-((3-chlorophenyl)amino...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase transforming protein Abl (Abelson murine leukemia virus) | BDBM3017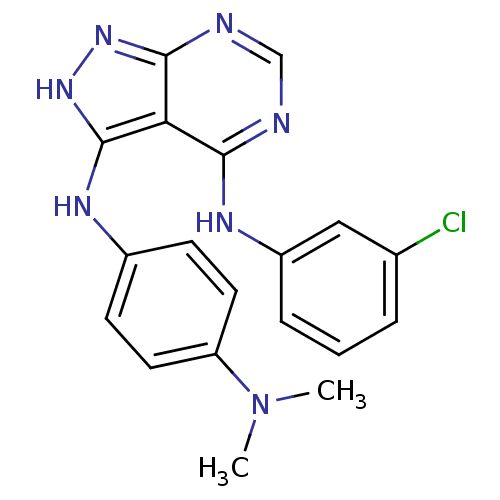 (3-((4-(Dimethylamino)phenyl)amino)-4-((3-chlorophe...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase transforming protein Abl (Abelson murine leukemia virus) | BDBM3015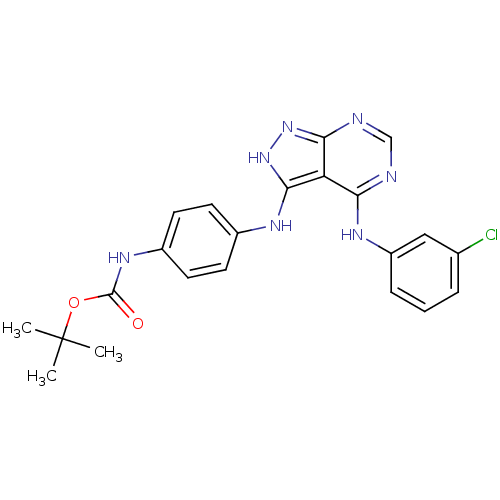 (3-((4-(N-BOC-amino)phenyl)amino)-4-((3-chloropheny...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase transforming protein Abl (Abelson murine leukemia virus) | BDBM3011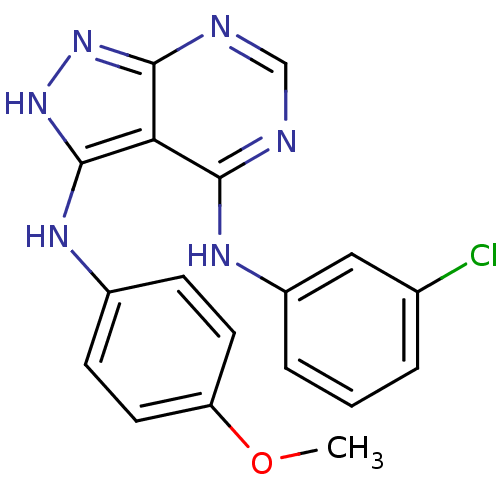 (3-((4-Methoxyphenyl)-amino)-4-((3-chlorophenyl)ami...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase transforming protein Abl (Abelson murine leukemia virus) | BDBM3003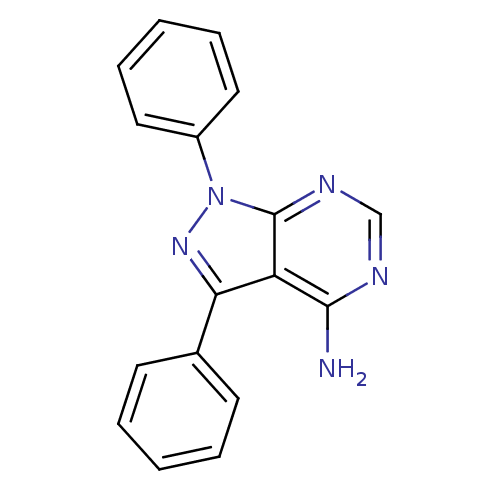 (1,3-diphenyl-1H-pyrazolo[3,4-d]pyrimidin-4-amine |...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.5 | 20 |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase transforming protein Abl (Abelson murine leukemia virus) | BDBM3026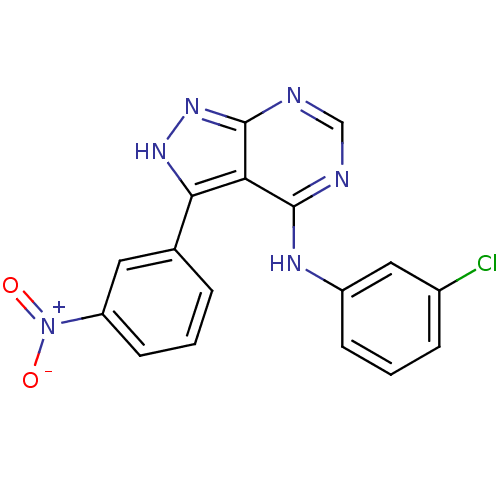 (3-(3-Nitrophenyl)-4-((3-chlorophenyl)amino)-1H-pyr...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase transforming protein Abl (Abelson murine leukemia virus) | BDBM50292494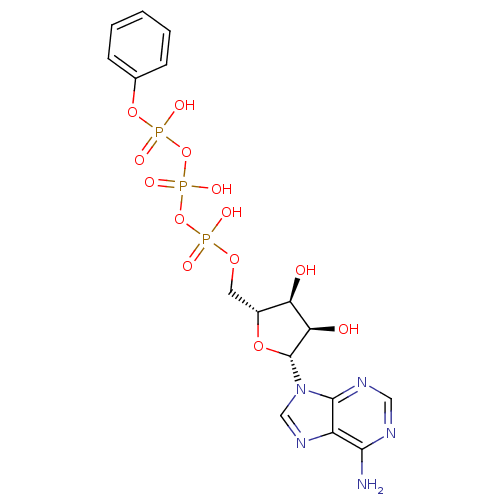 (CHEMBL485064 | {[(2R,3S,4R,5R)-5-(6-amino-9H-purin...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Abelson murine leukemia virus p60 v-abl | J Nat Prod 55: 1529-1560 (1992) Article DOI: 10.1021/np50089a001 BindingDB Entry DOI: 10.7270/Q2J966CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase transforming protein Abl (Abelson murine leukemia virus) | BDBM50292494 (CHEMBL485064 | {[(2R,3S,4R,5R)-5-(6-amino-9H-purin...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline& French Laboratories Curated by ChEMBL | Assay Description Inhibition of p60v-abl tyrosine kinase from Abelson murine leukemia virus | J Med Chem 31: 1768-72 (1988) BindingDB Entry DOI: 10.7270/Q2833SMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase transforming protein Abl (Abelson murine leukemia virus) | BDBM50292480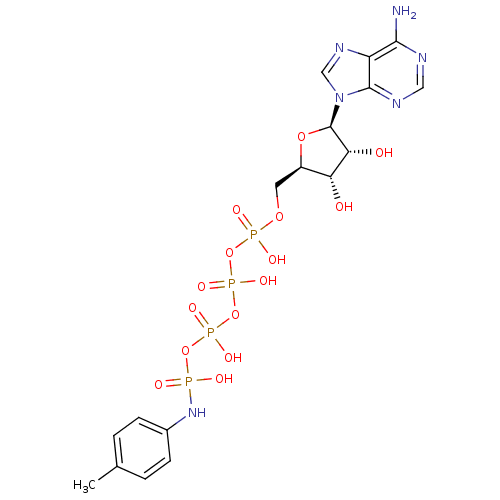 (CHEMBL445698 | {[(2R,3S,4R,5R)-5-(6-amino-9H-purin...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline& French Laboratories Curated by ChEMBL | Assay Description Inhibition of p60v-abl tyrosine kinase from Abelson murine leukemia virus | J Med Chem 31: 1768-72 (1988) BindingDB Entry DOI: 10.7270/Q2833SMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase transforming protein Abl (Abelson murine leukemia virus) | BDBM3024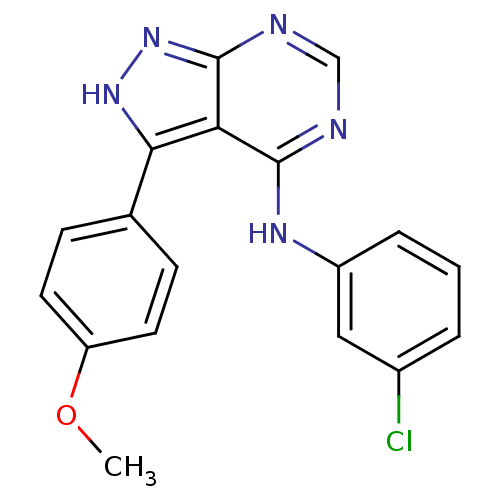 (3-(4-Methoxyphenyl)-4-((3-chlorophenyl)amino)-1H-p...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase transforming protein Abl (Abelson murine leukemia virus) | BDBM50292496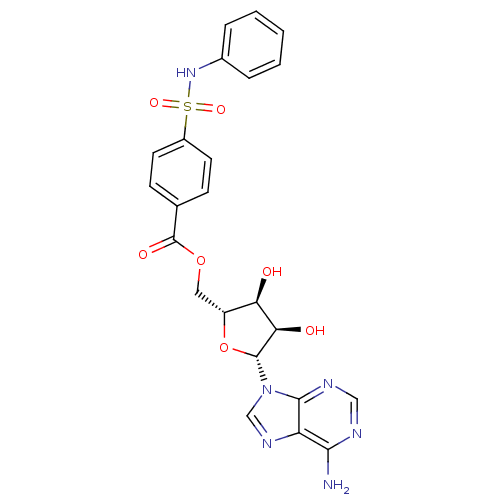 (((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihyd...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline& French Laboratories Curated by ChEMBL | Assay Description Inhibition of p60-vabl tyrosine kinase isolated from Abelson murine leukemia virus (A-MuLV) | J Med Chem 31: 1762-7 (1988) BindingDB Entry DOI: 10.7270/Q2CV4JB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase transforming protein Abl (Abelson murine leukemia virus) | BDBM50292481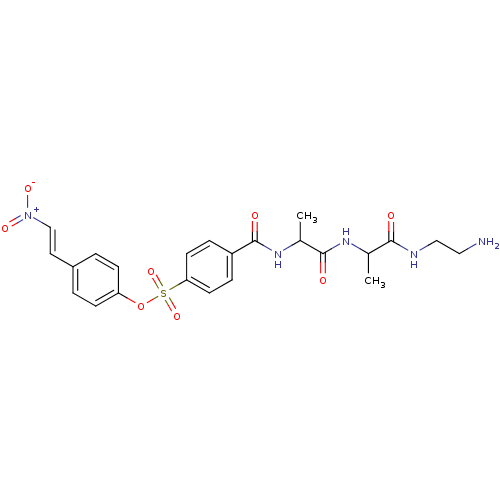 (4-(2-nitrovinyl)phenyl 4-((S)-1-((S)-1-(2-aminoeth...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Abelson murine leukemia virus p60 v-abl | J Nat Prod 55: 1529-1560 (1992) Article DOI: 10.1021/np50089a001 BindingDB Entry DOI: 10.7270/Q2J966CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase transforming protein Abl (Abelson murine leukemia virus) | BDBM50292480 (CHEMBL445698 | {[(2R,3S,4R,5R)-5-(6-amino-9H-purin...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Abelson murine leukemia virus p60 v-abl | J Nat Prod 55: 1529-1560 (1992) Article DOI: 10.1021/np50089a001 BindingDB Entry DOI: 10.7270/Q2J966CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase transforming protein Abl (Abelson murine leukemia virus) | BDBM50292496 (((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihyd...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Abelson murine leukemia virus p60 v-abl | J Nat Prod 55: 1529-1560 (1992) Article DOI: 10.1021/np50089a001 BindingDB Entry DOI: 10.7270/Q2J966CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase transforming protein Abl (Abelson murine leukemia virus) | BDBM50292482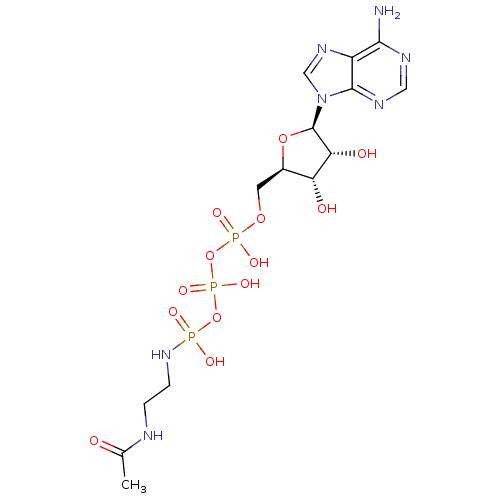 (({[({[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline& French Laboratories Curated by ChEMBL | Assay Description Inhibition of p60v-abl tyrosine kinase from Abelson murine leukemia virus | J Med Chem 31: 1768-72 (1988) BindingDB Entry DOI: 10.7270/Q2833SMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase transforming protein Abl (Abelson murine leukemia virus) | BDBM50007064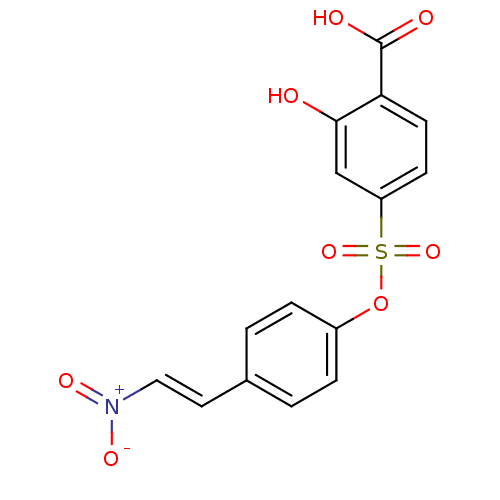 (2-Hydroxy-4-[4-(2-nitro-vinyl)-phenoxysulfonyl]-be...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Abelson murine leukemia virus p60 v-abl | J Nat Prod 55: 1529-1560 (1992) Article DOI: 10.1021/np50089a001 BindingDB Entry DOI: 10.7270/Q2J966CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase transforming protein Abl (Abelson murine leukemia virus) | BDBM50292482 (({[({[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Abelson murine leukemia virus p60 v-abl | J Nat Prod 55: 1529-1560 (1992) Article DOI: 10.1021/np50089a001 BindingDB Entry DOI: 10.7270/Q2J966CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase transforming protein Abl (Abelson murine leukemia virus) | BDBM50292484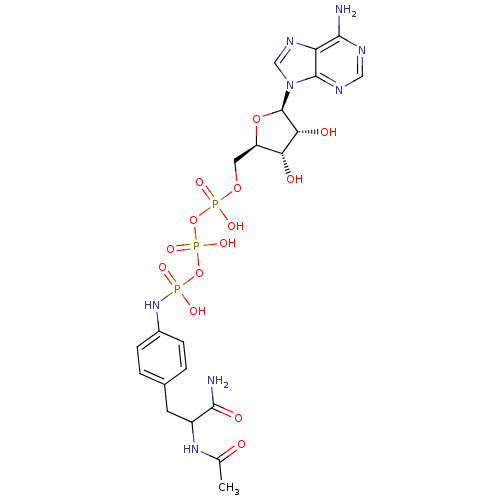 (CHEMBL509039 | {[(2R,3S,4R,5R)-5-(6-amino-9H-purin...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Abelson murine leukemia virus p60 v-abl | J Nat Prod 55: 1529-1560 (1992) Article DOI: 10.1021/np50089a001 BindingDB Entry DOI: 10.7270/Q2J966CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase transforming protein Abl (Abelson murine leukemia virus) | BDBM50007072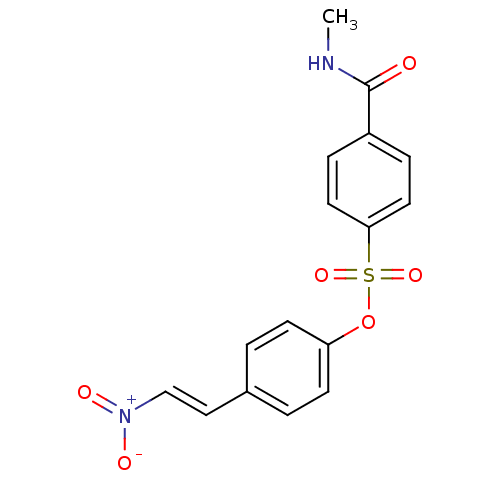 (4-(2-nitrovinyl)phenyl 4-(methylcarbamoyl)benzenes...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Abelson murine leukemia virus p60 v-abl | J Nat Prod 55: 1529-1560 (1992) Article DOI: 10.1021/np50089a001 BindingDB Entry DOI: 10.7270/Q2J966CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase transforming protein Abl (Abelson murine leukemia virus) | BDBM50292485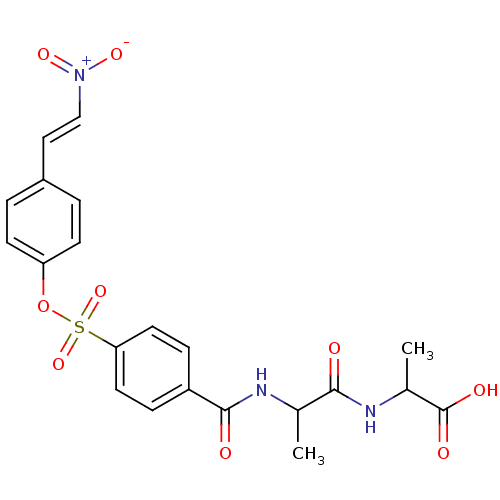 ((S)-2-((S)-2-(4-(4-(2-nitrovinyl)phenoxysulfonyl)b...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Abelson murine leukemia virus p60 v-abl | J Nat Prod 55: 1529-1560 (1992) Article DOI: 10.1021/np50089a001 BindingDB Entry DOI: 10.7270/Q2J966CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase transforming protein Abl (Abelson murine leukemia virus) | BDBM50007077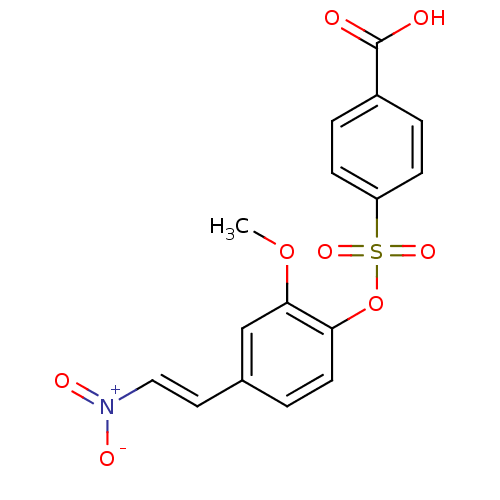 (4-(2-methoxy-4-(2-nitrovinyl)phenoxysulfonyl)benzo...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Abelson murine leukemia virus p60 v-abl | J Nat Prod 55: 1529-1560 (1992) Article DOI: 10.1021/np50089a001 BindingDB Entry DOI: 10.7270/Q2J966CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase transforming protein Abl (Abelson murine leukemia virus) | BDBM50292483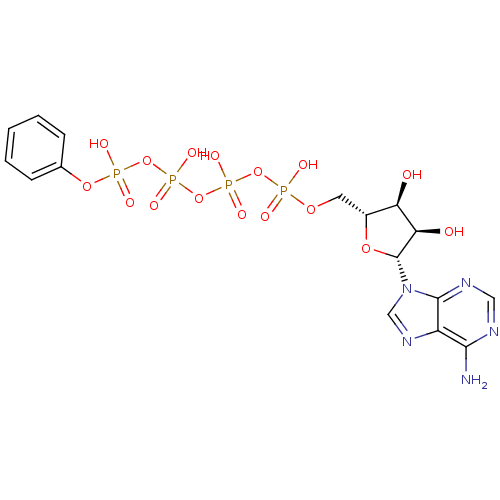 (CHEMBL520527 | {[(2R,3S,4R,5R)-5-(6-amino-9H-purin...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline& French Laboratories Curated by ChEMBL | Assay Description Inhibition of p60v-abl tyrosine kinase from Abelson murine leukemia virus | J Med Chem 31: 1768-72 (1988) BindingDB Entry DOI: 10.7270/Q2833SMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase transforming protein Abl (Abelson murine leukemia virus) | BDBM50292484 (CHEMBL509039 | {[(2R,3S,4R,5R)-5-(6-amino-9H-purin...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline& French Laboratories Curated by ChEMBL | Assay Description Inhibition of p60v-abl tyrosine kinase from Abelson murine leukemia virus | J Med Chem 31: 1768-72 (1988) BindingDB Entry DOI: 10.7270/Q2833SMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase transforming protein Abl (Abelson murine leukemia virus) | BDBM50292486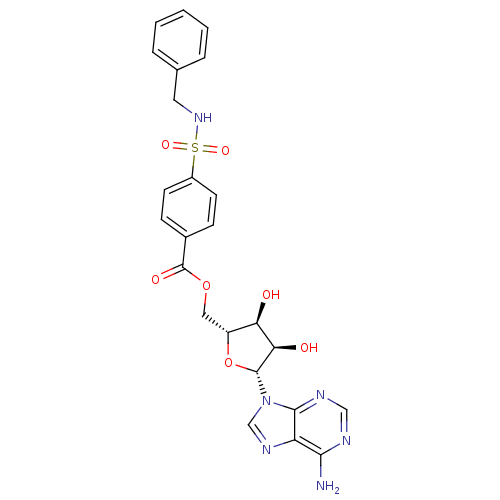 (((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihyd...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline& French Laboratories Curated by ChEMBL | Assay Description Inhibition of p60-vabl tyrosine kinase isolated from Abelson murine leukemia virus (A-MuLV) | J Med Chem 31: 1762-7 (1988) BindingDB Entry DOI: 10.7270/Q2CV4JB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase transforming protein Abl (Abelson murine leukemia virus) | BDBM50292487 (((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihyd...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline& French Laboratories Curated by ChEMBL | Assay Description Inhibition of p60-vabl tyrosine kinase isolated from Abelson murine leukemia virus (A-MuLV) | J Med Chem 31: 1762-7 (1988) BindingDB Entry DOI: 10.7270/Q2CV4JB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase transforming protein Abl (Abelson murine leukemia virus) | BDBM50292487 (((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihyd...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Abelson murine leukemia virus p60 v-abl | J Nat Prod 55: 1529-1560 (1992) Article DOI: 10.1021/np50089a001 BindingDB Entry DOI: 10.7270/Q2J966CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 80 total ) | Next | Last >> |