 Found 13 hits for monomerid = 50240701
Found 13 hits for monomerid = 50240701 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50240701
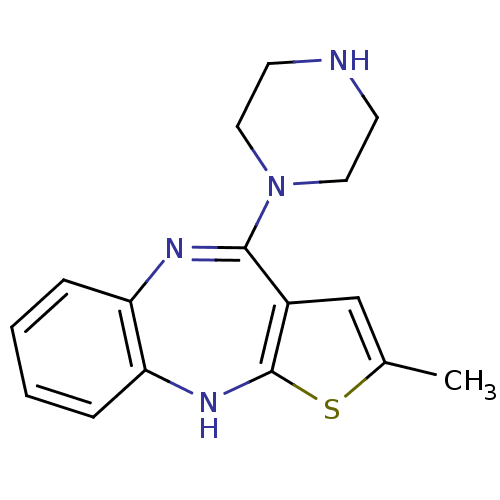
(2-Methyl-10-piperazin-1-yl-4H-3-thia-4,9-diaza-ben...)Show InChI InChI=1S/C16H18N4S/c1-11-10-12-15(20-8-6-17-7-9-20)18-13-4-2-3-5-14(13)19-16(12)21-11/h2-5,10,17,19H,6-9H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity of compound against neuronal Dopamine receptor D2 |
Bioorg Med Chem Lett 7: 25-30 (1997)
Article DOI: 10.1016/S0960-894X(96)00567-7
BindingDB Entry DOI: 10.7270/Q2KD1XXP |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50240701

(2-Methyl-10-piperazin-1-yl-4H-3-thia-4,9-diaza-ben...)Show InChI InChI=1S/C16H18N4S/c1-11-10-12-15(20-8-6-17-7-9-20)18-13-4-2-3-5-14(13)19-16(12)21-11/h2-5,10,17,19H,6-9H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity of compound against histamine H1 neuronal receptor |
Bioorg Med Chem Lett 7: 25-30 (1997)
Article DOI: 10.1016/S0960-894X(96)00567-7
BindingDB Entry DOI: 10.7270/Q2KD1XXP |
More data for this
Ligand-Target Pair | |
Serotonin 2 (5-HT2) receptor
(Homo sapiens (Human)) | BDBM50240701

(2-Methyl-10-piperazin-1-yl-4H-3-thia-4,9-diaza-ben...)Show InChI InChI=1S/C16H18N4S/c1-11-10-12-15(20-8-6-17-7-9-20)18-13-4-2-3-5-14(13)19-16(12)21-11/h2-5,10,17,19H,6-9H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity of compound against neuronal 5-hydroxytryptamine 2 receptor |
Bioorg Med Chem Lett 7: 25-30 (1997)
Article DOI: 10.1016/S0960-894X(96)00567-7
BindingDB Entry DOI: 10.7270/Q2KD1XXP |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50240701

(2-Methyl-10-piperazin-1-yl-4H-3-thia-4,9-diaza-ben...)Show InChI InChI=1S/C16H18N4S/c1-11-10-12-15(20-8-6-17-7-9-20)18-13-4-2-3-5-14(13)19-16(12)21-11/h2-5,10,17,19H,6-9H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 315: 1278-87 (2005)
Article DOI: 10.1124/jpet.105.092155
BindingDB Entry DOI: 10.7270/Q2Z60MNB |
More data for this
Ligand-Target Pair | |
Adrenergic receptor alpha-1
(Homo sapiens (Human)) | BDBM50240701

(2-Methyl-10-piperazin-1-yl-4H-3-thia-4,9-diaza-ben...)Show InChI InChI=1S/C16H18N4S/c1-11-10-12-15(20-8-6-17-7-9-20)18-13-4-2-3-5-14(13)19-16(12)21-11/h2-5,10,17,19H,6-9H2,1H3 | MMDB
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity of compound against Alpha-1 adrenergic receptor |
Bioorg Med Chem Lett 7: 25-30 (1997)
Article DOI: 10.1016/S0960-894X(96)00567-7
BindingDB Entry DOI: 10.7270/Q2KD1XXP |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50240701

(2-Methyl-10-piperazin-1-yl-4H-3-thia-4,9-diaza-ben...)Show InChI InChI=1S/C16H18N4S/c1-11-10-12-15(20-8-6-17-7-9-20)18-13-4-2-3-5-14(13)19-16(12)21-11/h2-5,10,17,19H,6-9H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 203 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity of compound against dopamine neuronal Dopamine receptor D1 |
Bioorg Med Chem Lett 7: 25-30 (1997)
Article DOI: 10.1016/S0960-894X(96)00567-7
BindingDB Entry DOI: 10.7270/Q2KD1XXP |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50240701

(2-Methyl-10-piperazin-1-yl-4H-3-thia-4,9-diaza-ben...)Show InChI InChI=1S/C16H18N4S/c1-11-10-12-15(20-8-6-17-7-9-20)18-13-4-2-3-5-14(13)19-16(12)21-11/h2-5,10,17,19H,6-9H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 315: 1278-87 (2005)
Article DOI: 10.1124/jpet.105.092155
BindingDB Entry DOI: 10.7270/Q2Z60MNB |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor
(Homo sapiens (Human)) | BDBM50240701

(2-Methyl-10-piperazin-1-yl-4H-3-thia-4,9-diaza-ben...)Show InChI InChI=1S/C16H18N4S/c1-11-10-12-15(20-8-6-17-7-9-20)18-13-4-2-3-5-14(13)19-16(12)21-11/h2-5,10,17,19H,6-9H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 333 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity of compound against muscarinic neuronal receptor |
Bioorg Med Chem Lett 7: 25-30 (1997)
Article DOI: 10.1016/S0960-894X(96)00567-7
BindingDB Entry DOI: 10.7270/Q2KD1XXP |
More data for this
Ligand-Target Pair | |
Adrenergic receptor alpha-2
(Homo sapiens (Human)) | BDBM50240701

(2-Methyl-10-piperazin-1-yl-4H-3-thia-4,9-diaza-ben...)Show InChI InChI=1S/C16H18N4S/c1-11-10-12-15(20-8-6-17-7-9-20)18-13-4-2-3-5-14(13)19-16(12)21-11/h2-5,10,17,19H,6-9H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity of compound against neuronal alpha-2 adrenergic receptor |
Bioorg Med Chem Lett 7: 25-30 (1997)
Article DOI: 10.1016/S0960-894X(96)00567-7
BindingDB Entry DOI: 10.7270/Q2KD1XXP |
More data for this
Ligand-Target Pair | |
Adrenergic receptor beta
(Homo sapiens (Human)) | BDBM50240701

(2-Methyl-10-piperazin-1-yl-4H-3-thia-4,9-diaza-ben...)Show InChI InChI=1S/C16H18N4S/c1-11-10-12-15(20-8-6-17-7-9-20)18-13-4-2-3-5-14(13)19-16(12)21-11/h2-5,10,17,19H,6-9H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity of compound against neuronal Beta adrenergic receptor |
Bioorg Med Chem Lett 7: 25-30 (1997)
Article DOI: 10.1016/S0960-894X(96)00567-7
BindingDB Entry DOI: 10.7270/Q2KD1XXP |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50240701

(2-Methyl-10-piperazin-1-yl-4H-3-thia-4,9-diaza-ben...)Show InChI InChI=1S/C16H18N4S/c1-11-10-12-15(20-8-6-17-7-9-20)18-13-4-2-3-5-14(13)19-16(12)21-11/h2-5,10,17,19H,6-9H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel in HEK293 cells by voltage-clamp method |
Eur J Med Chem 43: 2479-88 (2008)
Article DOI: 10.1016/j.ejmech.2007.12.025
BindingDB Entry DOI: 10.7270/Q2542PTB |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50240701

(2-Methyl-10-piperazin-1-yl-4H-3-thia-4,9-diaza-ben...)Show InChI InChI=1S/C16H18N4S/c1-11-10-12-15(20-8-6-17-7-9-20)18-13-4-2-3-5-14(13)19-16(12)21-11/h2-5,10,17,19H,6-9H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TCG Lifesciences Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Eur J Med Chem 46: 618-30 (2011)
Article DOI: 10.1016/j.ejmech.2010.11.042
BindingDB Entry DOI: 10.7270/Q2WQ052W |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50240701

(2-Methyl-10-piperazin-1-yl-4H-3-thia-4,9-diaza-ben...)Show InChI InChI=1S/C16H18N4S/c1-11-10-12-15(20-8-6-17-7-9-20)18-13-4-2-3-5-14(13)19-16(12)21-11/h2-5,10,17,19H,6-9H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel |
J Med Chem 52: 4266-76 (2009)
Article DOI: 10.1021/jm900002x
BindingDB Entry DOI: 10.7270/Q2MK6DT2 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data