 Found 10 hits for monomerid = 22875
Found 10 hits for monomerid = 22875 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM22875
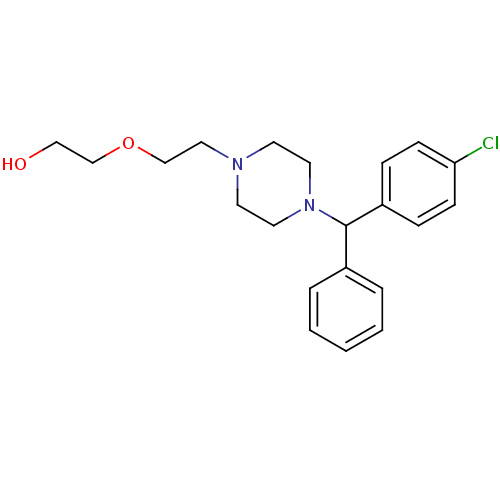
(2-(2-{4-[(4-chlorophenyl)(phenyl)methyl]piperazin-...)Show InChI InChI=1S/C21H27ClN2O2/c22-20-8-6-19(7-9-20)21(18-4-2-1-3-5-18)24-12-10-23(11-13-24)14-16-26-17-15-25/h1-9,21,25H,10-17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB S.A.
Curated by PDSP Ki Database
| |
Mol Pharmacol 61: 391-9 (2002)
Article DOI: 10.1124/mol.61.2.391
BindingDB Entry DOI: 10.7270/Q2D50KJ8 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM22875

(2-(2-{4-[(4-chlorophenyl)(phenyl)methyl]piperazin-...)Show InChI InChI=1S/C21H27ClN2O2/c22-20-8-6-19(7-9-20)21(18-4-2-1-3-5-18)24-12-10-23(11-13-24)14-16-26-17-15-25/h1-9,21,25H,10-17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB S.A.
Curated by PDSP Ki Database
| |
Mol Pharmacol 61: 391-9 (2002)
Article DOI: 10.1124/mol.61.2.391
BindingDB Entry DOI: 10.7270/Q2D50KJ8 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM22875

(2-(2-{4-[(4-chlorophenyl)(phenyl)methyl]piperazin-...)Show InChI InChI=1S/C21H27ClN2O2/c22-20-8-6-19(7-9-20)21(18-4-2-1-3-5-18)24-12-10-23(11-13-24)14-16-26-17-15-25/h1-9,21,25H,10-17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Binding affinity to histamine H1 receptor (unknown origin) |
Bioorg Med Chem Lett 28: 2039-2049 (2018)
Article DOI: 10.1016/j.bmcl.2018.04.059
BindingDB Entry DOI: 10.7270/Q2CJ8H4K |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM22875

(2-(2-{4-[(4-chlorophenyl)(phenyl)methyl]piperazin-...)Show InChI InChI=1S/C21H27ClN2O2/c22-20-8-6-19(7-9-20)21(18-4-2-1-3-5-18)24-12-10-23(11-13-24)14-16-26-17-15-25/h1-9,21,25H,10-17H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Binding affinity to 5-HT2A receptor (unknown origin) |
Bioorg Med Chem Lett 28: 2039-2049 (2018)
Article DOI: 10.1016/j.bmcl.2018.04.059
BindingDB Entry DOI: 10.7270/Q2CJ8H4K |
More data for this
Ligand-Target Pair | |
Alpha-1 Adrenergic Receptor/ adrenergic receptor/ adrenergic receptor
(Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM22875

(2-(2-{4-[(4-chlorophenyl)(phenyl)methyl]piperazin-...)Show InChI InChI=1S/C21H27ClN2O2/c22-20-8-6-19(7-9-20)21(18-4-2-1-3-5-18)24-12-10-23(11-13-24)14-16-26-17-15-25/h1-9,21,25H,10-17H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [3H]prazosin from rat cerebral cortex adrenergic receptor alpha1 by liquid scintillation counting method |
Bioorg Med Chem Lett 28: 2039-2049 (2018)
Article DOI: 10.1016/j.bmcl.2018.04.059
BindingDB Entry DOI: 10.7270/Q2CJ8H4K |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM22875

(2-(2-{4-[(4-chlorophenyl)(phenyl)methyl]piperazin-...)Show InChI InChI=1S/C21H27ClN2O2/c22-20-8-6-19(7-9-20)21(18-4-2-1-3-5-18)24-12-10-23(11-13-24)14-16-26-17-15-25/h1-9,21,25H,10-17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 378 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Binding affinity to D2 receptor (unknown origin) |
Bioorg Med Chem Lett 28: 2039-2049 (2018)
Article DOI: 10.1016/j.bmcl.2018.04.059
BindingDB Entry DOI: 10.7270/Q2CJ8H4K |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM22875

(2-(2-{4-[(4-chlorophenyl)(phenyl)methyl]piperazin-...)Show InChI InChI=1S/C21H27ClN2O2/c22-20-8-6-19(7-9-20)21(18-4-2-1-3-5-18)24-12-10-23(11-13-24)14-16-26-17-15-25/h1-9,21,25H,10-17H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | >-7.09 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Vrije Universiteit Amsterdam
| Assay Description
Ligand displacement assays were performed on The SK-N-MC/hH4R cell homogenates. Retained radioactivity was determined by liquid scintillation countin... |
J Pharmacol Exp Ther 314: 1310-21 (2005)
Article DOI: 10.1124/jpet.105.087965
BindingDB Entry DOI: 10.7270/Q2KD1W6V |
More data for this
Ligand-Target Pair | |
3C-like proteinase
(MERS-CoV) | BDBM22875

(2-(2-{4-[(4-chlorophenyl)(phenyl)methyl]piperazin-...)Show InChI InChI=1S/C21H27ClN2O2/c22-20-8-6-19(7-9-20)21(18-4-2-1-3-5-18)24-12-10-23(11-13-24)14-16-26-17-15-25/h1-9,21,25H,10-17H2 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.44E+4 | n/a | n/a | n/a | n/a |
University of Bonn
| Assay Description
This is a review article. |
Med Res Rev (2020)
Article DOI: 10.1002/med.21724
BindingDB Entry DOI: 10.7270/Q2JS9ST6 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type I I alpha subunit
(Homo sapiens (Human)) | BDBM22875

(2-(2-{4-[(4-chlorophenyl)(phenyl)methyl]piperazin-...)Show InChI InChI=1S/C21H27ClN2O2/c22-20-8-6-19(7-9-20)21(18-4-2-1-3-5-18)24-12-10-23(11-13-24)14-16-26-17-15-25/h1-9,21,25H,10-17H2 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of Batrachotoxinin [3H]BTX-B to high affinity sites on voltage dependent sodium channels in a vesicular preparation from guinea... |
J Med Chem 28: 381-8 (1985)
BindingDB Entry DOI: 10.7270/Q2Z321T8 |
More data for this
Ligand-Target Pair | |
Bile salt export pump
(Homo sapiens (Human)) | BDBM22875

(2-(2-{4-[(4-chlorophenyl)(phenyl)methyl]piperazin-...)Show InChI InChI=1S/C21H27ClN2O2/c22-20-8-6-19(7-9-20)21(18-4-2-1-3-5-18)24-12-10-23(11-13-24)14-16-26-17-15-25/h1-9,21,25H,10-17H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human BSEP expressed in baculovirus transfected fall armyworm Sf21 cell membranes vesicles assessed as reduction in ATP-dependent [3H]-... |
Drug Metab Dispos 40: 2332-41 (2012)
Article DOI: 10.1124/dmd.112.047068
BindingDB Entry DOI: 10.7270/Q2ZP488M |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data