

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50240480 (2S,3S)-3-({(2Z)-2-(2-ammonio-1,3-thiazol-4-yl)-2-[(1-carboxy-1-methylethoxy)imino]ethanoyl}amino)-2-methyl-4-oxoazetidine-1-sulfonate::(2S,3S)-3-({(2Z)-2-(2-ammonio-1,3-thiazol-4-yl)-2-[(1-carboxy-1-methylethoxy)imino]ethanoyl}amino)-2-methyl-4-oxoazetidine-1-sulfonate(aztreonam)::2-[1-(2-Amino-thiazol-4-yl)-1-((2S,3S)-2-methyl-4-oxo-1-sulfo-azetidin-3-ylcarbamoyl)-meth-(Z)-ylideneaminooxy]-2-methyl-propionic acid::AZTREONAM::Azactam::Azetreonam::CHEMBL158::SQ-26776
SMILES: C[C@H]1[C@H](NC(=O)C(=N/OC(C)(C)C(O)=O)\c2csc(N)n2)C(=O)N1S(O)(=O)=O
InChI Key: InChIKey=WZPBZJONDBGPKJ-VEHQQRBSSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-lactamase ADC-33 (Acinetobacter baumannii) | BDBM50240480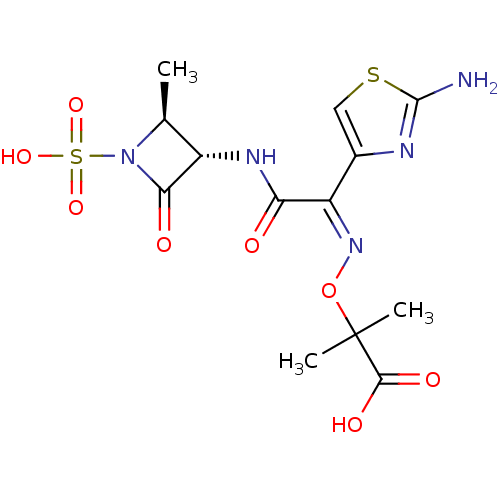 ((2S,3S)-3-({(2Z)-2-(2-ammonio-1,3-thiazol-4-yl)-2-...) | PDB UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Activity of Acinetobacter baumannii ADC-33 beta-lactamase | Antimicrob Agents Chemother 54: 3484-8 (2010) Article DOI: 10.1128/AAC.00050-10 BindingDB Entry DOI: 10.7270/Q2348KM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| PER-2 beta-lactamase (Citrobacter freundii) | BDBM50240480 ((2S,3S)-3-({(2Z)-2-(2-ammonio-1,3-thiazol-4-yl)-2-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Buenos Aires Curated by ChEMBL | Assay Description Inhibition of Citrobacter freundii PER2 beta lactamase | Antimicrob Agents Chemother 51: 2359-65 (2007) Article DOI: 10.1128/AAC.01395-06 BindingDB Entry DOI: 10.7270/Q24X57J4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ADC-11 (Acinetobacter baumannii) | BDBM50240480 ((2S,3S)-3-({(2Z)-2-(2-ammonio-1,3-thiazol-4-yl)-2-...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Activity of Acinetobacter baumannii ADC-11 beta-lactamase | Antimicrob Agents Chemother 54: 3484-8 (2010) Article DOI: 10.1128/AAC.00050-10 BindingDB Entry DOI: 10.7270/Q2348KM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| AmpC (Escherichia coli) | BDBM50240480 ((2S,3S)-3-({(2Z)-2-(2-ammonio-1,3-thiazol-4-yl)-2-...) | GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Basilea Pharmaceutica International Ltd. Curated by ChEMBL | Assay Description Inhibition of Escherichia coli ATCC 25922 AmpC | Antimicrob Agents Chemother 54: 2291-302 (2010) Article DOI: 10.1128/AAC.01525-09 BindingDB Entry DOI: 10.7270/Q20P1071 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Penicillin-binding protein 1A (Pseudomonas aeruginosa PAO1) | BDBM50240480 ((2S,3S)-3-({(2Z)-2-(2-ammonio-1,3-thiazol-4-yl)-2-...) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 3.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of fluorescent Bocillin FL from N-terminal His tagged Pseudomonas aeruginosa PAO1 PBP1a (residues 36 to 822) expressed in Escherichia co... | J Med Chem 57: 3845-55 (2014) Article DOI: 10.1021/jm500219c BindingDB Entry DOI: 10.7270/Q2SJ1N5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidoglycan D,D-transpeptidase FtsI (Pseudomonas aeruginosa) | BDBM50240480 ((2S,3S)-3-({(2Z)-2-(2-ammonio-1,3-thiazol-4-yl)-2-...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 68.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of bocillin FL binding to Pseudomonas aeruginosa PAO1 penicillin-binding protein 3 | Antimicrob Agents Chemother 51: 2621-4 (2007) Article DOI: 10.1128/aac.00029-07 BindingDB Entry DOI: 10.7270/Q2VH5RN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Efflux transporter (Salmonella newport) | BDBM50240480 ((2S,3S)-3-({(2Z)-2-(2-ammonio-1,3-thiazol-4-yl)-2-...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibition of Salmonella enterica serotype Newport AM17274 cephalosporinase CMY-31 by UV spectrophotometer in presence of 100 uM of cephalothin | Antimicrob Agents Chemother 53: 1256-9 (2009) Article DOI: 10.1128/AAC.01284-08 BindingDB Entry DOI: 10.7270/Q2V69JVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase ACC-4 (Escherichia coli) | BDBM50240480 ((2S,3S)-3-({(2Z)-2-(2-ammonio-1,3-thiazol-4-yl)-2-...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibition of Escherichia coli beta-lactamase ACC4 | Antimicrob Agents Chemother 51: 3763-7 (2007) Article DOI: 10.1128/AAC.00389-07 BindingDB Entry DOI: 10.7270/Q2222THB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidoglycan D,D-transpeptidase MrdA (Escherichia coli) | BDBM50240480 ((2S,3S)-3-({(2Z)-2-(2-ammonio-1,3-thiazol-4-yl)-2-...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >1.83E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of bocillin FL binding to Escherichia coli MC4100 penicillin-binding protein 2 | Antimicrob Agents Chemother 51: 2621-4 (2007) Article DOI: 10.1128/aac.00029-07 BindingDB Entry DOI: 10.7270/Q2VH5RN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Penicillin-binding protein 1B (Pseudomonas aeruginosa) | BDBM50240480 ((2S,3S)-3-({(2Z)-2-(2-ammonio-1,3-thiazol-4-yl)-2-...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 4.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of bocillin FL binding to Pseudomonas aeruginosa PAO1 penicillin-binding protein 1b | Antimicrob Agents Chemother 51: 2621-4 (2007) Article DOI: 10.1128/aac.00029-07 BindingDB Entry DOI: 10.7270/Q2VH5RN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidoglycan D,D-transpeptidase MrdA (Pseudomonas aeruginosa) | BDBM50240480 ((2S,3S)-3-({(2Z)-2-(2-ammonio-1,3-thiazol-4-yl)-2-...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 3.67E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of bocillin FL binding to Pseudomonas aeruginosa PAO1 penicillin-binding protein 2 | Antimicrob Agents Chemother 51: 2621-4 (2007) Article DOI: 10.1128/aac.00029-07 BindingDB Entry DOI: 10.7270/Q2VH5RN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Class C beta-lactamase CMY-36 (Klebsiella pneumoniae) | BDBM50240480 ((2S,3S)-3-({(2Z)-2-(2-ammonio-1,3-thiazol-4-yl)-2-...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibition of Klebsiella pneumoniae HP205 cephalosporinase CMY-36 by UV spectrophotometer in presence of 100 uM of cephalothin | Antimicrob Agents Chemother 53: 1256-9 (2009) Article DOI: 10.1128/AAC.01284-08 BindingDB Entry DOI: 10.7270/Q2V69JVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||