

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50019351 (-)-Codeine::(Codeine)::(codeine)10-methoxy-4-methyl-(1S,5R,13R,14S,17R)-12-oxa-4-azapentacyclo[9.6.1.01,13.05,17.07,18]octadeca-7(18),8,10,15-tetraen-14-ol::10-methoxy-4-methyl-(13R,14S)-12-oxa-4-azapentacyclo[9.6.1.01,13.05,17.07,18]octadeca-7(18),8,10-trien-14-ol(Dihydrocodeine)::10-methoxy-4-methyl-(1S,5R,13R,14S,17R)-12-oxa-4-azapentacyclo[9.6.1.01,13.05,17.07,18]octadeca-7(18),8,10,15-tetraen-14-ol::10-methoxy-4-methyl-(1S,5R,13R,14S,17R)-12-oxa-4-azapentacyclo[9.6.1.01,13.05,17.07,18]octadeca-7(18),8,10,15-tetraen-14-ol phosphate(codeine)::10-methoxy-4-methyl-(1S,5R,13R,14S,17R)-12-oxa-4-azapentacyclo[9.6.1.01,13.05,17.07,18]octadeca-7(18),8,10,15-tetraen-14-ol(codeine (H3PO4))::10-methoxy-4-methyl-(1S,5R,13R,14S,17R)-12-oxa-4-azapentacyclo[9.6.1.01,13.05,17.07,18]octadeca-7(18),8,10,15-tetraen-14-ol(codeine phosphate)::10-methoxy-4-methyl-(1S,5R,13R,14S,17R)-12-oxa-4-azapentacyclo[9.6.1.01,13.05,17.07,18]octadeca-7(18),8,10,15-tetraen-14-ol(codeine)::10-methoxy-4-methyl-(1S,5R,13R,14S,17R)-12-oxa-4-azapentacyclo[9.6.1.01,13.05,17.07,18]octadeca-7(18),8,10,15-tetraen-14-ol[codeine]::AMBENYL::BROMANYL::CODEINE::CODRIX::DIMETANE-DC::MYBANIL::TRIACIN-C
SMILES: COc1ccc2C[C@@H]3[C@@H]4C=C[C@H](O)[C@@H]5Oc1c2[C@]45CCN3C
InChI Key: InChIKey=OROGSEYTTFOCAN-DNJOTXNNSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mas-related G protein-coupled receptor X2 (MRGPRX2) (Homo sapiens (Human)) | BDBM50019351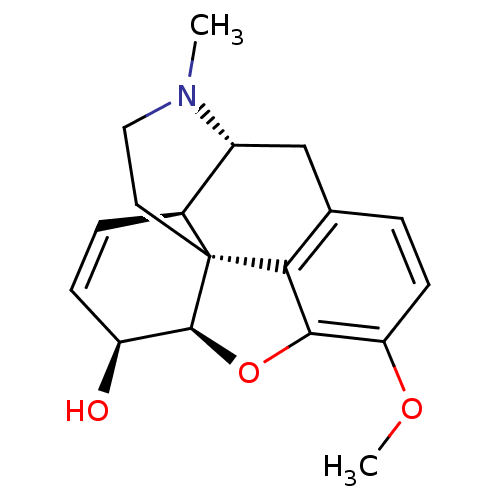 ((-)-Codeine | (Codeine) | (codeine)10-methoxy-4-me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.00E+4 | n/a | n/a | 7.4 | 30 |
University of North Carolina | Assay Description MRGPRX2 stable cells were maintained in DMEM containing 10% FBS, 100 μg/ml hygromycin B, and 15 μg/ml blasticidin. For the calcium mobiliza... | Nat Chem Biol 13: 529-536 (2017) Article DOI: 10.1038/nchembio.2334 BindingDB Entry DOI: 10.7270/Q20R9N82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier family 22 member 1 (Homo sapiens (Human)) | BDBM50019351 ((-)-Codeine | (Codeine) | (codeine)10-methoxy-4-me...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.36E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University Medicine Greifswald Curated by ChEMBL | Assay Description Inhibition of human OCT1 expressed in HEK293 cells assessed as reduction in ASP+ substrate uptake by microplate reader based analysis | J Med Chem 62: 9890-9905 (2019) Article DOI: 10.1021/acs.jmedchem.9b01301 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50019351 ((-)-Codeine | (Codeine) | (codeine)10-methoxy-4-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | >9.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description In vitro binding affinity against cloned human Opioid receptor delta 1 expressed in HEK 293S cells | J Med Chem 46: 34-48 (2002) Article DOI: 10.1021/jm020164l BindingDB Entry DOI: 10.7270/Q2TM7BTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D18 (Rattus norvegicus) | BDBM50019351 ((-)-Codeine | (Codeine) | (codeine)10-methoxy-4-me...) | GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.22E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Curated by ChEMBL | Assay Description Inhibition of MAMC O-dealkylation mediated by rat Cytochrome P450 2D4 expressed in Saccharomyces cerevisiae | J Med Chem 46: 74-86 (2002) Article DOI: 10.1021/jm0209578 BindingDB Entry DOI: 10.7270/Q2K64JSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50019351 ((-)-Codeine | (Codeine) | (codeine)10-methoxy-4-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description In vitro binding affinity against cloned human Opioid receptor kappa 1 expressed in HEK 293S cells | J Med Chem 46: 34-48 (2002) Article DOI: 10.1021/jm020164l BindingDB Entry DOI: 10.7270/Q2TM7BTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50019351 ((-)-Codeine | (Codeine) | (codeine)10-methoxy-4-me...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 105 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Tested for effective concentration against cloned human Opioid receptor mu 1 | J Med Chem 46: 34-48 (2002) Article DOI: 10.1021/jm020164l BindingDB Entry DOI: 10.7270/Q2TM7BTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D3 (Rattus norvegicus) | BDBM50019351 ((-)-Codeine | (Codeine) | (codeine)10-methoxy-4-me...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.09E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Curated by ChEMBL | Assay Description Inhibition of MAMC O-dealkylation mediated by rat Cytochrome P450 2D3 expressed in Saccharomyces cerevisiae | J Med Chem 46: 74-86 (2002) Article DOI: 10.1021/jm0209578 BindingDB Entry DOI: 10.7270/Q2K64JSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D1 (Rattus norvegicus) | BDBM50019351 ((-)-Codeine | (Codeine) | (codeine)10-methoxy-4-me...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.32E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Curated by ChEMBL | Assay Description Inhibition of MAMC O-dealkylation mediated by rat Cytochrome P450 2D1 expressed in Saccharomyces cerevisiae | J Med Chem 46: 74-86 (2002) Article DOI: 10.1021/jm0209578 BindingDB Entry DOI: 10.7270/Q2K64JSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D2 (Rattus norvegicus) | BDBM50019351 ((-)-Codeine | (Codeine) | (codeine)10-methoxy-4-me...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.38E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Curated by ChEMBL | Assay Description Inhibition of MAMC O-dealkylation mediated by rat Cytochrome P450 2D2 expressed in Saccharomyces cerevisiae | J Med Chem 46: 74-86 (2002) Article DOI: 10.1021/jm0209578 BindingDB Entry DOI: 10.7270/Q2K64JSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50019351 ((-)-Codeine | (Codeine) | (codeine)10-methoxy-4-me...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.04E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Curated by ChEMBL | Assay Description Inhibition of MAMC O-dealkylation mediated by human Cytochrome P450 2D6 expressed in human lymphoblastoid cell line | J Med Chem 46: 74-86 (2002) Article DOI: 10.1021/jm0209578 BindingDB Entry DOI: 10.7270/Q2K64JSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50019351 ((-)-Codeine | (Codeine) | (codeine)10-methoxy-4-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.02E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Reverse proteomics research institute Curated by ChEMBL | Assay Description Inhibitory concentration against potassium channel HERG | Bioorg Med Chem Lett 15: 2886-90 (2005) Article DOI: 10.1016/j.bmcl.2005.03.080 BindingDB Entry DOI: 10.7270/Q29S1S7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid receptor (Mus musculus (Mouse)-MOUSE) | BDBM50019351 ((-)-Codeine | (Codeine) | (codeine)10-methoxy-4-me...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required to inhibit [3H]naltrexone binding to Opioid receptors | J Med Chem 25: 1264-6 (1982) BindingDB Entry DOI: 10.7270/Q29P33VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid receptor (Rattus norvegicus (rat)-RAT) | BDBM50019351 ((-)-Codeine | (Codeine) | (codeine)10-methoxy-4-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description 50% Inhibition of stereospecific [3H]-naltrexone (10e-9 M) binding towards opiate receptor in rat brain homogenate | J Med Chem 28: 1177-80 (1985) BindingDB Entry DOI: 10.7270/Q2X350PW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid receptor (Rattus norvegicus (rat)-RAT) | BDBM50019351 ((-)-Codeine | (Codeine) | (codeine)10-methoxy-4-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required to inhibit stereospecific [3H]- naltrexone binding by 50% in rat brain homogenate | J Med Chem 27: 1219-22 (1984) BindingDB Entry DOI: 10.7270/Q2RJ4MPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid receptor (Rattus norvegicus (rat)) | BDBM50019351 ((-)-Codeine | (Codeine) | (codeine)10-methoxy-4-me...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 800 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity to opiate receptor in rat brain homogenates | J Med Chem 22: 328-31 (1979) Article DOI: 10.1021/jm00189a024 BindingDB Entry DOI: 10.7270/Q2GH9MX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mas-related G protein-coupled receptor X2 (MRGPRX2) (Homo sapiens (Human)) | BDBM50019351 ((-)-Codeine | (Codeine) | (codeine)10-methoxy-4-me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.80E+3 | n/a | n/a | 7.4 | 30 |
University of North Carolina | Assay Description HTLA cells (HEK-T cells stably expressing a β-arrestin2-TEV fusion protein and a tTa-dependent luciferase reporter) were maintained in DMEM (Cor... | Nat Chem Biol 13: 529-536 (2017) Article DOI: 10.1038/nchembio.2334 BindingDB Entry DOI: 10.7270/Q20R9N82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||