

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM23418 (2S)-5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-3,4-dihydro-2H-1-benzopyran-4-one::Hesperetin::Hesperitin
SMILES: COc1ccc(cc1O)[C@@H]1CC(=O)c2c(O)cc(O)cc2O1
InChI Key: InChIKey=AIONOLUJZLIMTK-AWEZNQCLSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM23418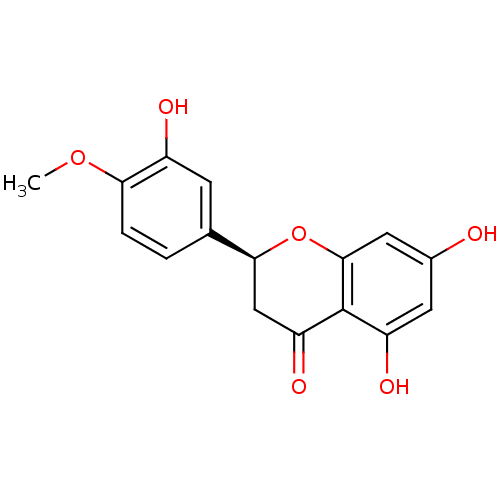 ((2S)-5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 3.30 | -11.6 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Aristotle University of Thessaloniki Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 7 preincubated for 15 mins at room temperature/6 hrs at 4 deg C by stopped-flow CO2 hydration assa... | Bioorg Med Chem 23: 7219-25 (2015) BindingDB Entry DOI: 10.7270/Q2862J84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM23418 ((2S)-5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 102 | -9.53 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Aristotle University of Thessaloniki Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 4 preincubated for 15 mins at room temperature/6 hrs at 4 deg C by stopped-flow CO2 hydration assa... | Bioorg Med Chem 23: 7219-25 (2015) BindingDB Entry DOI: 10.7270/Q2862J84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM23418 ((2S)-5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 454 | -8.65 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Aristotle University of Thessaloniki Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 12 preincubated for 15 mins at room temperature/6 hrs at 4 deg C by stopped-flow CO2 hydration ass... | Bioorg Med Chem 23: 7219-25 (2015) BindingDB Entry DOI: 10.7270/Q2862J84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM23418 ((2S)-5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | >1.00E+4 | >-6.82 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Aristotle University of Thessaloniki Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 2 preincubated for 15 mins at room temperature/6 hrs at 4 deg C by stopped-flow CO2 hydration assa... | Bioorg Med Chem 23: 7219-25 (2015) BindingDB Entry DOI: 10.7270/Q2862J84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM23418 ((2S)-5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | >1.00E+4 | >-6.82 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Aristotle University of Thessaloniki Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 1 preincubated for 15 mins at room temperature/6 hrs at 4 deg C by stopped-flow CO2 hydration assa... | Bioorg Med Chem 23: 7219-25 (2015) BindingDB Entry DOI: 10.7270/Q2862J84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM23418 ((2S)-5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.48E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE assessed as acetylthiocholine iodide hydrolysis after 10 mins preincubation by spectrophotometry | Bioorg Med Chem 20: 2595-602 (2012) Article DOI: 10.1016/j.bmc.2012.02.044 BindingDB Entry DOI: 10.7270/Q22808MC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Butyrylcholinesterase (BChE) (Equus caballus (Horse)) | BDBM23418 ((2S)-5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.77E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of equine BChE assessed as butyrylthiocholine iodide hydrolysis after 10 mins preincubation by spectrophotometry | Bioorg Med Chem 20: 2595-602 (2012) Article DOI: 10.1016/j.bmc.2012.02.044 BindingDB Entry DOI: 10.7270/Q22808MC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM23418 ((2S)-5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of DPP4 (unknown origin) using Gly-Pro-AMC as substrate preincubated for 4 secs followed by substrate addition and measured after 30 mins ... | Eur J Med Chem 151: 145-157 (2018) Article DOI: 10.1016/j.ejmech.2018.03.041 BindingDB Entry DOI: 10.7270/Q2CZ39SV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM23418 ((2S)-5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 7.88E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of LSD1 (unknown origin) by fluorescence assay | Bioorg Med Chem 27: 370-374 (2019) Article DOI: 10.1016/j.bmc.2018.12.013 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM23418 ((2S)-5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | Article | n/a | n/a | 2.69E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Homo sapiens (human) recombinant GSK3beta after 30 min by Kinase-Glo assay | Citation and Details Article DOI: 10.1007/s00044-012-0353-y BindingDB Entry DOI: 10.7270/Q29Z97T4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3C-like proteinase (3CL-PRO) (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM23418 ((2S)-5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-3...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 2.45E+4 | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Binding affinity to SARS coronavirus 3C-like protease by SPR analysis | Bioorg Med Chem 21: 3730-7 (2013) Article DOI: 10.1016/j.bmc.2013.04.026 BindingDB Entry DOI: 10.7270/Q24J0J2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3C-like proteinase (3CL-PRO) (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM23418 ((2S)-5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-3...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Inhibition of recombinant SARS coronavirus 3C-like protease trans-cleavage activity by ELISA | Bioorg Med Chem 21: 3730-7 (2013) Article DOI: 10.1016/j.bmc.2013.04.026 BindingDB Entry DOI: 10.7270/Q24J0J2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3C-like proteinase (3CL-PRO) (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM23418 ((2S)-5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-3...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Inhibition of SARS coronavirus 3C-like protease cis-cleavage activity transfected in african green monkey Vero cells by luciferase reporter gene assa... | Bioorg Med Chem 21: 3730-7 (2013) Article DOI: 10.1016/j.bmc.2013.04.026 BindingDB Entry DOI: 10.7270/Q24J0J2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM23418 ((2S)-5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 511 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Shizuoka Curated by ChEMBL | Assay Description Inhibition of human CYP1B1 by EROD assay | Bioorg Med Chem 18: 6310-5 (2010) Article DOI: 10.1016/j.bmc.2010.07.020 BindingDB Entry DOI: 10.7270/Q2GB248D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Homo sapiens (Human)) | BDBM23418 ((2S)-5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Shizuoka Curated by ChEMBL | Assay Description Inhibition of human CYP1A1 by EROD assay | Bioorg Med Chem 18: 6310-5 (2010) Article DOI: 10.1016/j.bmc.2010.07.020 BindingDB Entry DOI: 10.7270/Q2GB248D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A (Homo sapiens (Human)) | BDBM23418 ((2S)-5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-3...) | PDB MMDB Reactome pathway KEGG B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 3.46E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Shizuoka Curated by ChEMBL | Assay Description Inhibition of human CYP1A2 by EROD assay | Bioorg Med Chem 18: 6310-5 (2010) Article DOI: 10.1016/j.bmc.2010.07.020 BindingDB Entry DOI: 10.7270/Q2GB248D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL62 (Saccharomyces cerevisiae) | BDBM23418 ((2S)-5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-3...) | Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | Article | n/a | n/a | 1.50E+11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of baker's yeast alpha-glucosidase using p-nitrophenyl-alpha-D-glucopyranoside as substrate by spectrophotometry | Citation and Details Article DOI: 10.1007/s00044-011-9938-0 BindingDB Entry DOI: 10.7270/Q29W0JBG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-amylase (Homo sapiens (Human)) | BDBM23418 ((2S)-5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 6.0 | 22 |
Nestle Research Center | Assay Description The assay was carried out at room temperature for 10 min with salivary alpha-amylase, starch, and test compounds. The reducing sugar was determined b... | J Med Chem 51: 3555-61 (2008) Article DOI: 10.1021/jm800115x BindingDB Entry DOI: 10.7270/Q237771Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM23418 ((2S)-5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5.46E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Eberhard Karls University of Tuebingen | Assay Description The p38alpha reaction was carried out by using kinase (12ng per well), ATP (100uM) and incubated for 60 min at 37 C. For the JNK3 assay, kinase (10n... | Chembiochem 11: 2579-88 (2010) Article DOI: 10.1002/cbic.201000487 BindingDB Entry DOI: 10.7270/Q21C1VDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||