

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50214817 CHEMBL236757::N6-aminoadenosine
SMILES: NNc1ncnc2n(cnc12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O
InChI Key: InChIKey=DGKZTAGCCXJUAT-KQYNXXCUSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine Receptors A2a (A2a) (Rattus norvegicus (rat)) | BDBM50214817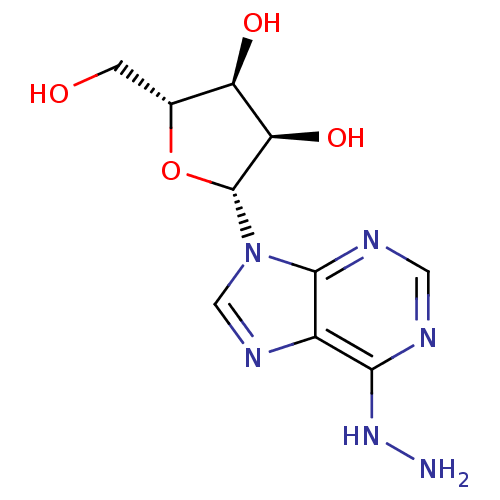 (CHEMBL236757 | N6-aminoadenosine) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article | 7.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-CGS- 21680 from Adenosine A2 receptor of rat striatal membranes | Bioorg Med Chem Lett 7: 3085-3090 (1997) Article DOI: 10.1016/S0960-894X(97)10177-9 BindingDB Entry DOI: 10.7270/Q2SQ90W2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine Receptors A2a (A2a) (Rattus norvegicus (rat)) | BDBM50214817 (CHEMBL236757 | N6-aminoadenosine) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Binding affinity to adenosine A2A receptor in rat striatal membranes by measuring displacement of specific [3H]-CGS- 21680 as radioligand | J Med Chem 38: 1174-88 (1995) BindingDB Entry DOI: 10.7270/Q2HQ40KN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a and A3 (Rattus norvegicus) | BDBM50214817 (CHEMBL236757 | N6-aminoadenosine) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.69E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Binding affinity determined by displacement of specific binding of [125I]N-(4-amino-3-iodophenethyl)-adenosine in membranes of CHO cells stably trans... | J Med Chem 38: 1174-88 (1995) BindingDB Entry DOI: 10.7270/Q2HQ40KN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50214817 (CHEMBL236757 | N6-aminoadenosine) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.97E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Binding affinity to adenosine A1 receptor in rat brain membranes by measuring displacement of specific [3H]PIA as radioligand. | J Med Chem 38: 1174-88 (1995) BindingDB Entry DOI: 10.7270/Q2HQ40KN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50214817 (CHEMBL236757 | N6-aminoadenosine) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article | 2.97E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity at Adenosine A1 receptor in rat brain membranes by [3H](R)-PIA displacement. | Bioorg Med Chem Lett 7: 3085-3090 (1997) Article DOI: 10.1016/S0960-894X(97)10177-9 BindingDB Entry DOI: 10.7270/Q2SQ90W2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase M1 chain/Ribonucleotide reductase (Homo sapiens (Human)) | BDBM50214817 (CHEMBL236757 | N6-aminoadenosine) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.44E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Camerino Curated by ChEMBL | Assay Description Inhibition of human recombinant ribonucleotide reductase p53R2/M1 expressed in Escherichia coli BL21 (DE3) after 30 mins by [3H]CDP reduction method | Eur J Med Chem 46: 1499-504 (2011) Article DOI: 10.1016/j.ejmech.2011.01.055 BindingDB Entry DOI: 10.7270/Q2K074KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RRM1/RRM2 holoenzyme (Homo sapiens (Human)) | BDBM50214817 (CHEMBL236757 | N6-aminoadenosine) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.32E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Camerino Curated by ChEMBL | Assay Description Inhibition of human recombinant ribonucleotide reductase M2/M1 expressed in Escherichia coli BL21 (DE3) after 30 mins by [3H]CDP reduction method | Eur J Med Chem 46: 1499-504 (2011) Article DOI: 10.1016/j.ejmech.2011.01.055 BindingDB Entry DOI: 10.7270/Q2K074KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||