

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50068561 (16E,24E,26E,28E)-1,18-Dihydroxy-12-[2-(4-hydroxy-3-methoxy-cyclohexyl)-1-methyl-ethyl]-19,30-dimethoxy-15,17,21,23,29,35-hexamethyl-11,36-dioxa-4-aza-tricyclo[30.3.1.0*4,9*]hexatriaconta-16,24,26,28-tetraene-2::1,18-Dihydroxy-12-[2-(4-hydroxy-3-methoxy-cyclohexyl)-1-methyl-ethyl]-19,30,33-trimethoxy-15,17,21,23,29,35-hexamethyl-11,36-dioxa-4-aza-tricyclo[30.3.1.0*4,9*]hexatriaconta-16,24,26,28-tetraene-2,3,10,14,20-pentaone;Rapamycin::1,18-dihydroxy-12-{2-[4-hydroxy-3-methoxy-(4R)-cyclohexyl]-1-methylethyl}-19,30-dimethoxy-15,17,21,23,29,35-hexamethyl-11,36-dioxa-4-azatricyclo[30.3.1.04,9]hexatriaconta-16,24,26,28-tetraene-2,3,10,14,20-pentaone::AY-22989::CHEMBL413::FK-506-M::RAPAMYCIN IMMUNOSUPPRESSANT DRUG::Rapamune::Rapamycin::SIROLIMUS::Sirolimus analogue::WY-090217
SMILES: CO[C@@H]1C[C@H](CC(C)[C@@H]2CC(=O)[C@H](C)\C=C(C)\[C@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@H](C[C@H]3O[C@](O)([C@H](C)C[C@@H]3OC)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)OC)CC[C@H]1O
InChI Key: InChIKey=HJLWEROPDBIBIJ-KRBMXJRHSA-N
Data: 2 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50068561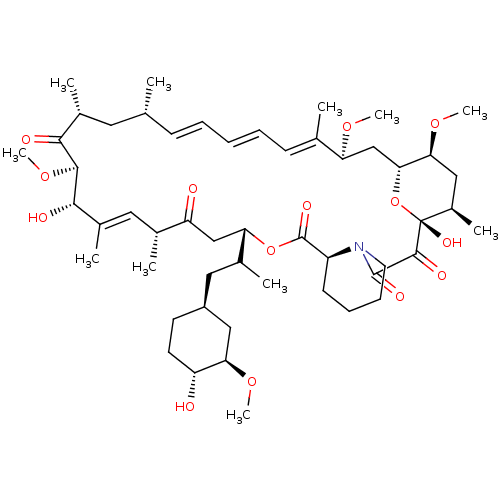 ((16E,24E,26E,28E)-1,18-Dihydroxy-12-[2-(4-hydroxy-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against FKBP12 | Bioorg Med Chem Lett 15: 5340-3 (2005) Article DOI: 10.1016/j.bmcl.2005.06.106 BindingDB Entry DOI: 10.7270/Q2V40TRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50068561 ((16E,24E,26E,28E)-1,18-Dihydroxy-12-[2-(4-hydroxy-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | MMDB PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description The compound was tested for binding afifnity to FK506 binding protein 12 with an ascomycin conjugate of alkaline phosphatase in a competition binding... | Bioorg Med Chem Lett 10: 1405-8 (2000) BindingDB Entry DOI: 10.7270/Q2CV4GZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||