

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50347620 RIFAXIMIN::Rifacol::Rifaxidin
SMILES: CO[C@H]1\C=C\O[C@@]2(C)Oc3c(C2=O)c2c4nc5cc(C)ccn5c4c(NC(=O)\C(C)=C/C=C/[C@H](C)[C@H](O)[C@@H](C)[C@@H](O)[C@@H](C)[C@H](OC(C)=O)[C@@H]1C)c(O)c2c(O)c3C
InChI Key: InChIKey=NZCRJKRKKOLAOJ-XRCRFVBUSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pregnane X receptor (Homo sapiens (Human)) | BDBM50347620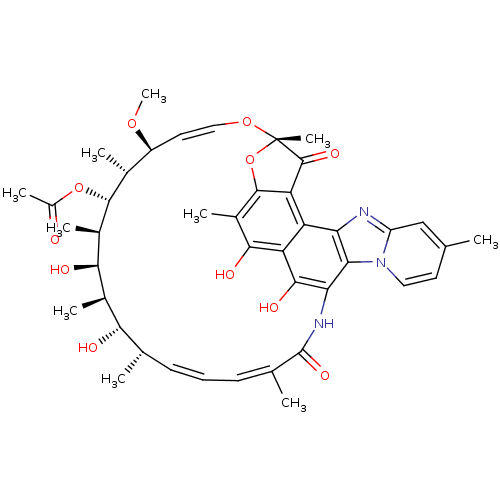 (RIFAXIMIN | Rifacol | Rifaxidin) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | 1.41E+4 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Activation of human PXR expressed in human HepG2 (DPX-2) cells after 24 hrs by luciferase reporter gene based luminescent analysis | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pregnane X receptor (Homo sapiens (Human)) | BDBM50347620 (RIFAXIMIN | Rifacol | Rifaxidin) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | 1.41E+4 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Activation of human PXR expressed in human HepG2 (DPX-2) cells assessed as induction of CYP3A4 after 24 hrs by luminescent analysis | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pregnane X receptor (Homo sapiens (Human)) | BDBM50347620 (RIFAXIMIN | Rifacol | Rifaxidin) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank PC cid PC sid PDB UniChem Patents | PubMed | n/a | n/a | n/a | n/a | 1.12E+4 | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Transactivation of full length human PXR transfected in human HepG2 cells after 18 hrs by luciferase reporter assay relative to rifaximin | Eur J Med Chem 103: 551-62 (2015) BindingDB Entry DOI: 10.7270/Q2N87CMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pregnane X receptor (Homo sapiens (Human)) | BDBM50347620 (RIFAXIMIN | Rifacol | Rifaxidin) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a |
Universita di Napoli Federico II Curated by ChEMBL | Assay Description Transactivation of human PXR expressed in HepG2 cells after 18 hrs by luciferase reporter gene assay | J Med Chem 54: 4590-9 (2011) Article DOI: 10.1021/jm200241s BindingDB Entry DOI: 10.7270/Q2W37WP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Multidrug resistance-associated protein 4 (Homo sapiens (Human)) | BDBM50347620 (RIFAXIMIN | Rifacol | Rifaxidin) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank PC cid PC sid PDB UniChem Patents | PubMed | n/a | n/a | 3.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc Curated by ChEMBL | Assay Description Inhibition of human MRP4 overexpressed in Sf9 cell membrane vesicles assessed as uptake of [3H]-estradiol-17beta-D-glucuronide in presence of ATP and... | Toxicol Sci 136: 216-41 (2013) BindingDB Entry DOI: 10.7270/Q2JM2D2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Canalicular multispecific organic anion transporter 1 (Homo sapiens (Human)) | BDBM50347620 (RIFAXIMIN | Rifacol | Rifaxidin) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank PC cid PC sid PDB UniChem Patents | PubMed | n/a | n/a | 1.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc Curated by ChEMBL | Assay Description Inhibition of human MRP2 overexpressed in Sf9 cell membrane vesicles assessed as uptake of [3H]-estradiol-17beta-D-glucuronide in presence of ATP and... | Toxicol Sci 136: 216-41 (2013) BindingDB Entry DOI: 10.7270/Q2JM2D2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Canalicular multispecific organic anion transporter 2 (Homo sapiens (Human)) | BDBM50347620 (RIFAXIMIN | Rifacol | Rifaxidin) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank PC cid PC sid PDB UniChem Patents | PubMed | n/a | n/a | 6.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc Curated by ChEMBL | Assay Description Inhibition of human MRP3 overexpressed in Sf9 insect cell membrane vesicles assessed as uptake of [3H]-estradiol-17beta-D-glucuronide in presence of ... | Toxicol Sci 136: 216-41 (2013) BindingDB Entry DOI: 10.7270/Q2JM2D2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile salt export pump (Homo sapiens (Human)) | BDBM50347620 (RIFAXIMIN | Rifacol | Rifaxidin) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank PC cid PC sid PDB UniChem Patents | PubMed | n/a | n/a | 4.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc Curated by ChEMBL | Assay Description Inhibition of human BSEP overexpressed in Sf9 cell membrane vesicles assessed as uptake of [3H]-taurocholate in presence of ATP measured after 15 to ... | Toxicol Sci 136: 216-41 (2013) BindingDB Entry DOI: 10.7270/Q2JM2D2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pregnane X receptor (Homo sapiens (Human)) | BDBM50347620 (RIFAXIMIN | Rifacol | Rifaxidin) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a |
Universit£ di Napoli"Federico II" Curated by ChEMBL | Assay Description Agonist activity at human full-length PXR transfected in human HepG2 cells co-transfected with pSG5-RXR assessed as induction of transactivation by d... | Eur J Med Chem 73: 126-34 (2014) Article DOI: 10.1016/j.ejmech.2013.12.005 BindingDB Entry DOI: 10.7270/Q2H70H9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||