

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM11542 (1S,3S,5S)-2-[(2S)-2-amino-2-(3-hydroxyadamantan-1-yl)acetyl]-2-azabicyclo[3.1.0]hexane-3-carbonitrile; 2,2,2-trifluoroacetic acid::(S)-3-Hydroxyadamantylglycine-L-cis-4,5-methanoprolinenitrile TFA salt::BMS-477118::Saxagliptin
SMILES: N[C@H](C(=O)N1[C@H]2C[C@H]2C[C@H]1C#N)C12CC3CC(CC(O)(C3)C1)C2
InChI Key: InChIKey=QGJUIPDUBHWZPV-SGTAVMJGSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11542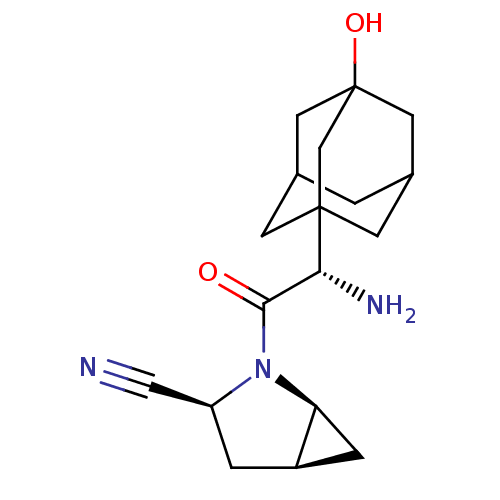 ((1S,3S,5S)-2-[(2S)-2-amino-2-(3-hydroxyadamantan-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 0.600 | -12.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... | J Med Chem 48: 5025-37 (2005) Article DOI: 10.1021/jm050261p BindingDB Entry DOI: 10.7270/Q2FN14DM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Multidrug resistance-associated protein 4 (Homo sapiens (Human)) | BDBM11542 ((1S,3S,5S)-2-[(2S)-2-amino-2-(3-hydroxyadamantan-1...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 1.33E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc Curated by ChEMBL | Assay Description Inhibition of human MRP4 overexpressed in Sf9 cell membrane vesicles assessed as uptake of [3H]-estradiol-17beta-D-glucuronide in presence of ATP and... | Toxicol Sci 136: 216-41 (2013) BindingDB Entry DOI: 10.7270/Q2JM2D2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile salt export pump (Homo sapiens (Human)) | BDBM11542 ((1S,3S,5S)-2-[(2S)-2-amino-2-(3-hydroxyadamantan-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 1.33E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc Curated by ChEMBL | Assay Description Inhibition of human BSEP overexpressed in Sf9 cell membrane vesicles assessed as uptake of [3H]-taurocholate in presence of ATP measured after 15 to ... | Toxicol Sci 136: 216-41 (2013) BindingDB Entry DOI: 10.7270/Q2JM2D2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Canalicular multispecific organic anion transporter 2 (Homo sapiens (Human)) | BDBM11542 ((1S,3S,5S)-2-[(2S)-2-amino-2-(3-hydroxyadamantan-1...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 1.33E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc Curated by ChEMBL | Assay Description Inhibition of human MRP3 overexpressed in Sf9 insect cell membrane vesicles assessed as uptake of [3H]-estradiol-17beta-D-glucuronide in presence of ... | Toxicol Sci 136: 216-41 (2013) BindingDB Entry DOI: 10.7270/Q2JM2D2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Canalicular multispecific organic anion transporter 1 (Homo sapiens (Human)) | BDBM11542 ((1S,3S,5S)-2-[(2S)-2-amino-2-(3-hydroxyadamantan-1...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 1.33E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc Curated by ChEMBL | Assay Description Inhibition of human MRP2 overexpressed in Sf9 cell membrane vesicles assessed as uptake of [3H]-estradiol-17beta-D-glucuronide in presence of ATP and... | Toxicol Sci 136: 216-41 (2013) BindingDB Entry DOI: 10.7270/Q2JM2D2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||