 Found 17 hits for monomerid = 50013111
Found 17 hits for monomerid = 50013111 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Thymidine kinase, cytosolic
(Homo sapiens (Human)) | BDBM50013111
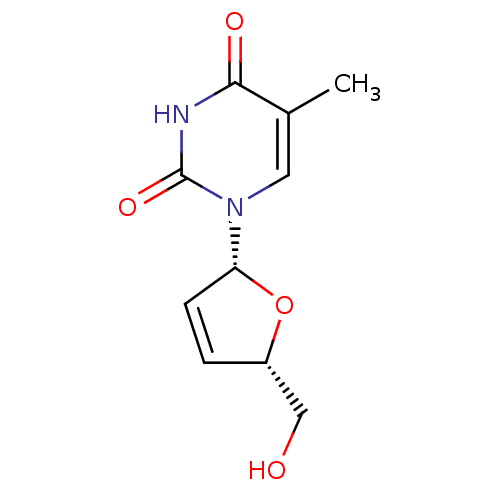
(1-((2R,5S)-5-(hydroxymethyl)-2,5-dihydrofuran-2-yl...)Show SMILES Cc1cn([C@@H]2O[C@H](CO)C=C2)c(=O)[nH]c1=O |r,c:9| Show InChI InChI=1S/C10H12N2O4/c1-6-4-12(10(15)11-9(6)14)8-3-2-7(5-13)16-8/h2-4,7-8,13H,5H2,1H3,(H,11,14,15)/t7-,8+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 5.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Michigan Cancer Foundation
Curated by ChEMBL
| Assay Description
Inhibitory affect against rabbit thymus thymidine kinase |
J Med Chem 33: 258-63 (1990)
BindingDB Entry DOI: 10.7270/Q2P26ZQS |
More data for this
Ligand-Target Pair | |
Thymidine kinase, cytosolic
(Homo sapiens (Human)) | BDBM50013111

(1-((2R,5S)-5-(hydroxymethyl)-2,5-dihydrofuran-2-yl...)Show SMILES Cc1cn([C@@H]2O[C@H](CO)C=C2)c(=O)[nH]c1=O |r,c:9| Show InChI InChI=1S/C10H12N2O4/c1-6-4-12(10(15)11-9(6)14)8-3-2-7(5-13)16-8/h2-4,7-8,13H,5H2,1H3,(H,11,14,15)/t7-,8+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 6.15E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Michigan Cancer Foundation
Curated by ChEMBL
| Assay Description
Inhibitory affect against rabbit thymus thymidine kinase |
J Med Chem 33: 258-63 (1990)
BindingDB Entry DOI: 10.7270/Q2P26ZQS |
More data for this
Ligand-Target Pair | |
Thymidine kinase
(Rattus norvegicus) | BDBM50013111

(1-((2R,5S)-5-(hydroxymethyl)-2,5-dihydrofuran-2-yl...)Show SMILES Cc1cn([C@@H]2O[C@H](CO)C=C2)c(=O)[nH]c1=O |r,c:9| Show InChI InChI=1S/C10H12N2O4/c1-6-4-12(10(15)11-9(6)14)8-3-2-7(5-13)16-8/h2-4,7-8,13H,5H2,1H3,(H,11,14,15)/t7-,8+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.70E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition against rat cytoplasmic Thymidine kinase |
J Med Chem 25: 644-9 (1982)
BindingDB Entry DOI: 10.7270/Q2GQ6ZBJ |
More data for this
Ligand-Target Pair | |
Thymidine kinase 2
(Rattus norvegicus) | BDBM50013111

(1-((2R,5S)-5-(hydroxymethyl)-2,5-dihydrofuran-2-yl...)Show SMILES Cc1cn([C@@H]2O[C@H](CO)C=C2)c(=O)[nH]c1=O |r,c:9| Show InChI InChI=1S/C10H12N2O4/c1-6-4-12(10(15)11-9(6)14)8-3-2-7(5-13)16-8/h2-4,7-8,13H,5H2,1H3,(H,11,14,15)/t7-,8+/m0/s1 | Reactome pathway
KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3.25E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition against rat mitochondrial thymidine kinase |
J Med Chem 25: 644-9 (1982)
BindingDB Entry DOI: 10.7270/Q2GQ6ZBJ |
More data for this
Ligand-Target Pair | |
Human immunodeficiency virus type 1 reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50013111

(1-((2R,5S)-5-(hydroxymethyl)-2,5-dihydrofuran-2-yl...)Show SMILES Cc1cn([C@@H]2O[C@H](CO)C=C2)c(=O)[nH]c1=O |r,c:9| Show InChI InChI=1S/C10H12N2O4/c1-6-4-12(10(15)11-9(6)14)8-3-2-7(5-13)16-8/h2-4,7-8,13H,5H2,1H3,(H,11,14,15)/t7-,8+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | n/a | n/a | 450 | n/a | n/a | n/a | n/a |
The University of Georgia
Curated by ChEMBL
| Assay Description
In vitro antiviral activity against HIV-1 Reverse transcriptase wild type |
Bioorg Med Chem Lett 13: 1993-6 (2003)
BindingDB Entry DOI: 10.7270/Q2639Q86 |
More data for this
Ligand-Target Pair | |
Human immunodeficiency virus type 1 reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50013111

(1-((2R,5S)-5-(hydroxymethyl)-2,5-dihydrofuran-2-yl...)Show SMILES Cc1cn([C@@H]2O[C@H](CO)C=C2)c(=O)[nH]c1=O |r,c:9| Show InChI InChI=1S/C10H12N2O4/c1-6-4-12(10(15)11-9(6)14)8-3-2-7(5-13)16-8/h2-4,7-8,13H,5H2,1H3,(H,11,14,15)/t7-,8+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | n/a | n/a | 50 | n/a | n/a | n/a | n/a |
URA CNRS 1309
Curated by ChEMBL
| Assay Description
Effective concentration that reduces HIV-induced cytopathic effect by 50% was determined by reverse transcriptase (RT) assay. |
J Med Chem 36: 826-30 (1993)
BindingDB Entry DOI: 10.7270/Q2WW7JBD |
More data for this
Ligand-Target Pair | |
Human immunodeficiency virus type 1 reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50013111

(1-((2R,5S)-5-(hydroxymethyl)-2,5-dihydrofuran-2-yl...)Show SMILES Cc1cn([C@@H]2O[C@H](CO)C=C2)c(=O)[nH]c1=O |r,c:9| Show InChI InChI=1S/C10H12N2O4/c1-6-4-12(10(15)11-9(6)14)8-3-2-7(5-13)16-8/h2-4,7-8,13H,5H2,1H3,(H,11,14,15)/t7-,8+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | n/a | n/a | 500 | n/a | n/a | n/a | n/a |
The University of Georgia
Curated by ChEMBL
| Assay Description
In vitro antiviral activity against HIV-1 Reverse transcriptase M184V mutant |
Bioorg Med Chem Lett 13: 1993-6 (2003)
BindingDB Entry DOI: 10.7270/Q2639Q86 |
More data for this
Ligand-Target Pair | |
Canalicular multispecific organic anion transporter 2
(Homo sapiens (Human)) | BDBM50013111

(1-((2R,5S)-5-(hydroxymethyl)-2,5-dihydrofuran-2-yl...)Show SMILES Cc1cn([C@@H]2O[C@H](CO)C=C2)c(=O)[nH]c1=O |r,c:9| Show InChI InChI=1S/C10H12N2O4/c1-6-4-12(10(15)11-9(6)14)8-3-2-7(5-13)16-8/h2-4,7-8,13H,5H2,1H3,(H,11,14,15)/t7-,8+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.33E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of human MRP3 overexpressed in Sf9 insect cell membrane vesicles assessed as uptake of [3H]-estradiol-17beta-D-glucuronide in presence of ... |
Toxicol Sci 136: 216-41 (2013)
BindingDB Entry DOI: 10.7270/Q2JM2D2D |
More data for this
Ligand-Target Pair | |
Canalicular multispecific organic anion transporter 1
(Homo sapiens (Human)) | BDBM50013111

(1-((2R,5S)-5-(hydroxymethyl)-2,5-dihydrofuran-2-yl...)Show SMILES Cc1cn([C@@H]2O[C@H](CO)C=C2)c(=O)[nH]c1=O |r,c:9| Show InChI InChI=1S/C10H12N2O4/c1-6-4-12(10(15)11-9(6)14)8-3-2-7(5-13)16-8/h2-4,7-8,13H,5H2,1H3,(H,11,14,15)/t7-,8+/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.33E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of human MRP2 overexpressed in Sf9 cell membrane vesicles assessed as uptake of [3H]-estradiol-17beta-D-glucuronide in presence of ATP and... |
Toxicol Sci 136: 216-41 (2013)
BindingDB Entry DOI: 10.7270/Q2JM2D2D |
More data for this
Ligand-Target Pair | |
Multidrug resistance-associated protein 4
(Homo sapiens (Human)) | BDBM50013111

(1-((2R,5S)-5-(hydroxymethyl)-2,5-dihydrofuran-2-yl...)Show SMILES Cc1cn([C@@H]2O[C@H](CO)C=C2)c(=O)[nH]c1=O |r,c:9| Show InChI InChI=1S/C10H12N2O4/c1-6-4-12(10(15)11-9(6)14)8-3-2-7(5-13)16-8/h2-4,7-8,13H,5H2,1H3,(H,11,14,15)/t7-,8+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.33E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of human MRP4 overexpressed in Sf9 cell membrane vesicles assessed as uptake of [3H]-estradiol-17beta-D-glucuronide in presence of ATP and... |
Toxicol Sci 136: 216-41 (2013)
BindingDB Entry DOI: 10.7270/Q2JM2D2D |
More data for this
Ligand-Target Pair | |
Bile salt export pump
(Homo sapiens (Human)) | BDBM50013111

(1-((2R,5S)-5-(hydroxymethyl)-2,5-dihydrofuran-2-yl...)Show SMILES Cc1cn([C@@H]2O[C@H](CO)C=C2)c(=O)[nH]c1=O |r,c:9| Show InChI InChI=1S/C10H12N2O4/c1-6-4-12(10(15)11-9(6)14)8-3-2-7(5-13)16-8/h2-4,7-8,13H,5H2,1H3,(H,11,14,15)/t7-,8+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.33E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of human BSEP overexpressed in Sf9 cell membrane vesicles assessed as uptake of [3H]-taurocholate in presence of ATP measured after 15 to ... |
Toxicol Sci 136: 216-41 (2013)
BindingDB Entry DOI: 10.7270/Q2JM2D2D |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50013111

(1-((2R,5S)-5-(hydroxymethyl)-2,5-dihydrofuran-2-yl...)Show SMILES Cc1cn([C@@H]2O[C@H](CO)C=C2)c(=O)[nH]c1=O |r,c:9| Show InChI InChI=1S/C10H12N2O4/c1-6-4-12(10(15)11-9(6)14)8-3-2-7(5-13)16-8/h2-4,7-8,13H,5H2,1H3,(H,11,14,15)/t7-,8+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase |
Bioorg Med Chem 15: 5519-28 (2007)
Article DOI: 10.1016/j.bmc.2007.05.047
BindingDB Entry DOI: 10.7270/Q20P12S1 |
More data for this
Ligand-Target Pair | |
Serum albumin
(Homo sapiens (Human)) | BDBM50013111

(1-((2R,5S)-5-(hydroxymethyl)-2,5-dihydrofuran-2-yl...)Show SMILES Cc1cn([C@@H]2O[C@H](CO)C=C2)c(=O)[nH]c1=O |r,c:9| Show InChI InChI=1S/C10H12N2O4/c1-6-4-12(10(15)11-9(6)14)8-3-2-7(5-13)16-8/h2-4,7-8,13H,5H2,1H3,(H,11,14,15)/t7-,8+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a |
Mercer University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity to human serum albumin with excitation at 280 nm after 2 hrs by spectrofluorimetric analysis |
Bioorg Med Chem Lett 25: 3168-71 (2015)
BindingDB Entry DOI: 10.7270/Q21N82W0 |
More data for this
Ligand-Target Pair | |
exonuclease V (RecBCD complex), alpha chain
(Escherichia coli str. K-12 substr. MG1655) | BDBM50013111

(1-((2R,5S)-5-(hydroxymethyl)-2,5-dihydrofuran-2-yl...)Show SMILES Cc1cn([C@@H]2O[C@H](CO)C=C2)c(=O)[nH]c1=O |r,c:9| Show InChI InChI=1S/C10H12N2O4/c1-6-4-12(10(15)11-9(6)14)8-3-2-7(5-13)16-8/h2-4,7-8,13H,5H2,1H3,(H,11,14,15)/t7-,8+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PCBioAssay
| n/a | n/a | 5.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
| |
PubChem Bioassay (2013)
BindingDB Entry DOI: 10.7270/Q21G0JXG |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50013111

(1-((2R,5S)-5-(hydroxymethyl)-2,5-dihydrofuran-2-yl...)Show SMILES Cc1cn([C@@H]2O[C@H](CO)C=C2)c(=O)[nH]c1=O |r,c:9| Show InChI InChI=1S/C10H12N2O4/c1-6-4-12(10(15)11-9(6)14)8-3-2-7(5-13)16-8/h2-4,7-8,13H,5H2,1H3,(H,11,14,15)/t7-,8+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase-mediated thymidine incorporation into D23/D36 primer-template preincubated for 15 mins by polyacrylamide gel... |
Bioorg Med Chem 18: 4661-73 (2010)
Article DOI: 10.1016/j.bmc.2010.05.025
BindingDB Entry DOI: 10.7270/Q2NS0XQ1 |
More data for this
Ligand-Target Pair | |
exonuclease V (RecBCD complex), alpha chain
(Escherichia coli str. K-12 substr. MG1655) | BDBM50013111

(1-((2R,5S)-5-(hydroxymethyl)-2,5-dihydrofuran-2-yl...)Show SMILES Cc1cn([C@@H]2O[C@H](CO)C=C2)c(=O)[nH]c1=O |r,c:9| Show InChI InChI=1S/C10H12N2O4/c1-6-4-12(10(15)11-9(6)14)8-3-2-7(5-13)16-8/h2-4,7-8,13H,5H2,1H3,(H,11,14,15)/t7-,8+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PCBioAssay
| n/a | n/a | 6.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
| |
PubChem Bioassay (2013)
BindingDB Entry DOI: 10.7270/Q21G0JXG |
More data for this
Ligand-Target Pair | |
exonuclease V (RecBCD complex), alpha chain
(Escherichia coli str. K-12 substr. MG1655) | BDBM50013111

(1-((2R,5S)-5-(hydroxymethyl)-2,5-dihydrofuran-2-yl...)Show SMILES Cc1cn([C@@H]2O[C@H](CO)C=C2)c(=O)[nH]c1=O |r,c:9| Show InChI InChI=1S/C10H12N2O4/c1-6-4-12(10(15)11-9(6)14)8-3-2-7(5-13)16-8/h2-4,7-8,13H,5H2,1H3,(H,11,14,15)/t7-,8+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PCBioAssay
| n/a | n/a | 7.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
| |
PubChem Bioassay (2013)
BindingDB Entry DOI: 10.7270/Q21G0JXG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data