

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50407398 ABIRATERONE ACETATE::CB7630::Zytiga
SMILES: CC(=O)O[C@H]1CC[C@]2(C)[C@H]3CC[C@@]4(C)[C@@H](CC=C4c4cccnc4)[C@@H]3CC=C2C1
InChI Key: InChIKey=UVIQSJCZCSLXRZ-UBUQANBQSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 17A1 (Homo sapiens (Human)) | BDBM50407398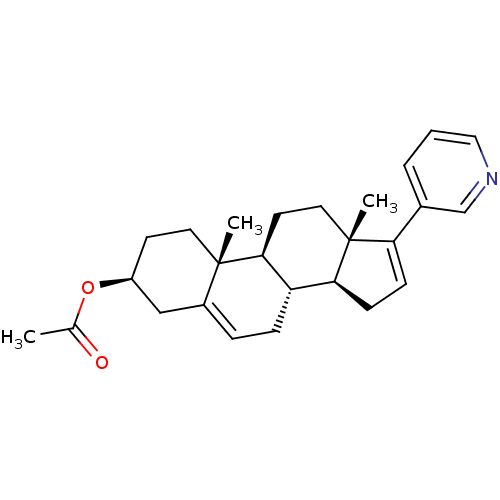 (ABIRATERONE ACETATE | CB7630 | Zytiga) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Ability to inhibit the C17,20-lyase enzyme by 50% using 17-alpha-hydroxyprogesterone as substrate. | J Med Chem 38: 2463-71 (1995) BindingDB Entry DOI: 10.7270/Q20K27KR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen Receptor (Homo sapiens (Human)) | BDBM50407398 (ABIRATERONE ACETATE | CB7630 | Zytiga) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.71E+4 | n/a | n/a | n/a | n/a |
Drexel University College of Medicine Curated by ChEMBL | Assay Description Antagonist activity at full length human androgen receptor expressed in mammalian expression system measured after 22 to 24 hrs by luciferase reporte... | Bioorg Med Chem Lett 27: 2216-2220 (2017) Article DOI: 10.1016/j.bmcl.2017.03.030 BindingDB Entry DOI: 10.7270/Q2P84F1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 17A1 (Homo sapiens (Human)) | BDBM50407398 (ABIRATERONE ACETATE | CB7630 | Zytiga) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Ability to inhibit the Steroid 17-alpha-hydroxylase/17,20 lyase enzyme by 50%. | J Med Chem 38: 2463-71 (1995) BindingDB Entry DOI: 10.7270/Q20K27KR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||