

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: CC(C)[C@H](NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)c1ccccc1)C(C)(C)C)C(=O)C(=O)NC1CCCCC1
InChI Key: InChIKey=YGCFQCIVTNJIRZ-AVCGJXAMSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peptidase 1 (Dermatophagoides pteronyssinus (European house dus...) | BDBM103020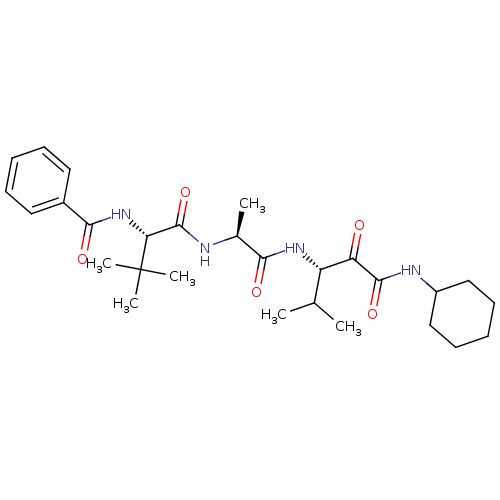 (US8541363, PVA-026) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
St George's Hosptial Medical School; The University of Manchester US Patent | Assay Description The fluorogenic substrate used for measuring Der p 1 proteolytic activity was 2-aminobenzoylvalylalanylnorleucylseryl-(3-nitro)tyrosinyl aspartamide.... | US Patent US8541363 (2013) BindingDB Entry DOI: 10.7270/Q2PC3105 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidase 1 (Dermatophagoides pteronyssinus (European house dus...) | BDBM103020 (US8541363, PVA-026) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Domainex Ltd. Curated by ChEMBL | Assay Description Inhibition of house dust mite Derp-1 after 20 mins | J Med Chem 57: 9447-62 (2014) Article DOI: 10.1021/jm501102h BindingDB Entry DOI: 10.7270/Q2R212ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM103020 (US8541363, PVA-026) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 378 | n/a | n/a | n/a | n/a | n/a | n/a |
Domainex Ltd. Curated by ChEMBL | Assay Description Inhibition of human liver cathepsin B using ABz-Gly-Ile-Val-Arg-Ala-Lys-DNP-OH as substrate after 10 mins by fluorescence assay | J Med Chem 57: 9447-62 (2014) Article DOI: 10.1021/jm501102h BindingDB Entry DOI: 10.7270/Q2R212ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||