

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM10309 5-(pyrrolidine-1-sulfonyl)-2,3-dihydro-1H-indole-2,3-dione::5-[1-(Pyrrolidinyl)sulfonyl]isatin::Isatin Sulfonamide 20
SMILES: O=C1Nc2ccc(cc2C1=O)S(=O)(=O)N1CCCC1
InChI Key: InChIKey=QKCWUYHXHMXOLG-UHFFFAOYSA-N
PDB links: 1 PDB ID contains this monomer as substructures. 1 PDB ID contains inhibitors having a similarity of 90% to this monomer.
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Caspase-3 (Homo sapiens (Human)) | BDBM10309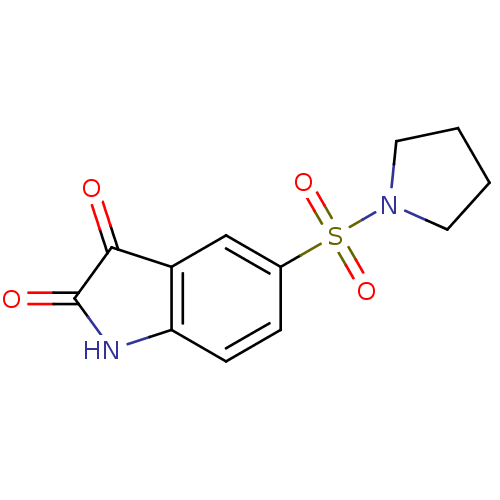 (5-(pyrrolidine-1-sulfonyl)-2,3-dihydro-1H-indole-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40E+3 | -8.11 | 2.80E+3 | n/a | n/a | n/a | n/a | 7.5 | 30 |
GlaxoSmithKline | Assay Description The substrate peptides terminating in AMC/AFC are processed by caspases with or without inhibitors, and the accumulation of AMC/AFC was assessed with... | J Med Chem 44: 2015-26 (2001) Article DOI: 10.1021/jm0100537 BindingDB Entry DOI: 10.7270/Q2ZW1J42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-7 (Homo sapiens (Human)) | BDBM10309 (5-(pyrrolidine-1-sulfonyl)-2,3-dihydro-1H-indole-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The substrate peptides terminating in AMC/AFC are processed by caspases with or without inhibitors, and the accumulation of AMC/AFC was assessed with... | J Med Chem 44: 2015-26 (2001) Article DOI: 10.1021/jm0100537 BindingDB Entry DOI: 10.7270/Q2ZW1J42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-1 (Homo sapiens (Human)) | BDBM10309 (5-(pyrrolidine-1-sulfonyl)-2,3-dihydro-1H-indole-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The substrate peptides terminating in AMC/AFC are processed by caspases with or without inhibitors, and the accumulation of AMC/AFC was assessed with... | J Med Chem 44: 2015-26 (2001) Article DOI: 10.1021/jm0100537 BindingDB Entry DOI: 10.7270/Q2ZW1J42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-9 (Homo sapiens (Human)) | BDBM10309 (5-(pyrrolidine-1-sulfonyl)-2,3-dihydro-1H-indole-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The substrate peptides terminating in AMC/AFC are processed by caspases with or without inhibitors, and the accumulation of AMC/AFC was assessed with... | J Med Chem 44: 2015-26 (2001) Article DOI: 10.1021/jm0100537 BindingDB Entry DOI: 10.7270/Q2ZW1J42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-8 (Homo sapiens (Human)) | BDBM10309 (5-(pyrrolidine-1-sulfonyl)-2,3-dihydro-1H-indole-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The substrate peptides terminating in AMC/AFC are processed by caspases with or without inhibitors, and the accumulation of AMC/AFC was assessed with... | J Med Chem 44: 2015-26 (2001) Article DOI: 10.1021/jm0100537 BindingDB Entry DOI: 10.7270/Q2ZW1J42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-4 (Homo sapiens (Human)) | BDBM10309 (5-(pyrrolidine-1-sulfonyl)-2,3-dihydro-1H-indole-2...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The substrate peptides terminating in AMC/AFC are processed by caspases with or without inhibitors, and the accumulation of AMC/AFC was assessed with... | J Med Chem 44: 2015-26 (2001) Article DOI: 10.1021/jm0100537 BindingDB Entry DOI: 10.7270/Q2ZW1J42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM10309 (5-(pyrrolidine-1-sulfonyl)-2,3-dihydro-1H-indole-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The substrate peptides terminating in AMC/AFC are processed by caspases with or without inhibitors, and the accumulation of AMC/AFC was assessed with... | J Med Chem 44: 2015-26 (2001) Article DOI: 10.1021/jm0100537 BindingDB Entry DOI: 10.7270/Q2ZW1J42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-6 (Homo sapiens (Human)) | BDBM10309 (5-(pyrrolidine-1-sulfonyl)-2,3-dihydro-1H-indole-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The substrate peptides terminating in AMC/AFC are processed by caspases with or without inhibitors, and the accumulation of AMC/AFC was assessed with... | J Med Chem 44: 2015-26 (2001) Article DOI: 10.1021/jm0100537 BindingDB Entry DOI: 10.7270/Q2ZW1J42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM10309 (5-(pyrrolidine-1-sulfonyl)-2,3-dihydro-1H-indole-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Osmania University College for Women Curated by ChEMBL | Assay Description Inhibition of caspase 3 in human SK-N-SH cells assessed as accumulation of fluorogenic 7-amino-4-methyl coumarin by flurometric assay | Bioorg Med Chem 17: 6040-7 (2009) Article DOI: 10.1016/j.bmc.2009.06.069 BindingDB Entry DOI: 10.7270/Q2CC10R2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||