

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM103841 5172-mcP1P3
SMILES: CCc1nc2ccc(OC)cc2nc1O[C@@H]1C[C@@H]2N(C1)C(=O)[C@H](CCCCC\C=C/[C@@H]1C[C@]1(NC2=O)C(=O)NS(=O)(=O)C1CC1)NC(=O)OC(C)(C)C
InChI Key: InChIKey=YCYCBCSJYGZXLL-LUTRIBIWSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hepatitis C virus serine protease, NS3/NS4A (Hepatitis C virus) | BDBM103841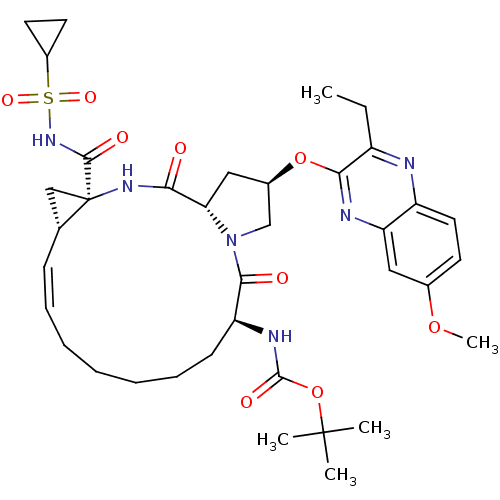 (5172-mcP1P3) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Inhibition of wild type HCV genotype 1a NS3/4A protease expressed in Escherichia coli BL21(DE3) using Ac-DE-Dap(QXL 520)-EE-Abu-psi-[COO]AS-C(5-FAMsp... | ACS Med Chem Lett 9: 691-696 (2018) Article DOI: 10.1021/acsmedchemlett.8b00150 BindingDB Entry DOI: 10.7270/Q29C7125 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatitis C virus serine protease, NS3/NS4A (Hepatitis C virus) | BDBM103841 (5172-mcP1P3) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1a NS3/4A protease R155K mutant expressed in Escherichia coli BL21(DE3) using Ac-DE-Dap(QXL 520)-EE-Abu-psi-[COO]AS-C(5-FA... | ACS Med Chem Lett 9: 691-696 (2018) Article DOI: 10.1021/acsmedchemlett.8b00150 BindingDB Entry DOI: 10.7270/Q29C7125 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatitis C virus serine protease, NS3/NS4A (Hepatitis C virus) | BDBM103841 (5172-mcP1P3) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1a NS3/4A protease D168A mutant expressed in Escherichia coli BL21(DE3) using Ac-DE-Dap(QXL 520)-EE-Abu-psi-[COO]AS-C(5-FA... | ACS Med Chem Lett 9: 691-696 (2018) Article DOI: 10.1021/acsmedchemlett.8b00150 BindingDB Entry DOI: 10.7270/Q29C7125 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HCV NS3-NS4A Serine Proteinase (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM103841 (5172-mcP1P3) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School | Assay Description NS3/4A protease was preincubated with increasing concentration of drugs in protease reaction buffer for 1 hour. Inhibition assays were performed in ... | ACS Chem Biol 8: 1469-78 (2013) Article DOI: 10.1021/cb400100g BindingDB Entry DOI: 10.7270/Q2FQ9V7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||