

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM10440 (5S)-5-[(12-{[(5S)-2-oxo-1,2,5,6,7,8-hexahydroquinolin-5-yl]amino}dodecyl)amino]-1,2,5,6,7,8-hexahydroquinolin-2-one::(S,S)-(-)-bis(12)-hupyridone
SMILES: O=c1ccc2[C@H](CCCc2[nH]1)NCCCCCCCCCCCCN[C@H]1CCCc2[nH]c(=O)ccc12
InChI Key: InChIKey=VFFGYPZORQBRNM-UIOOFZCWSA-N
PDB links: 1 PDB ID matches this monomer. 1 PDB ID contains inhibitors having a similarity of 90% to this monomer.
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM10440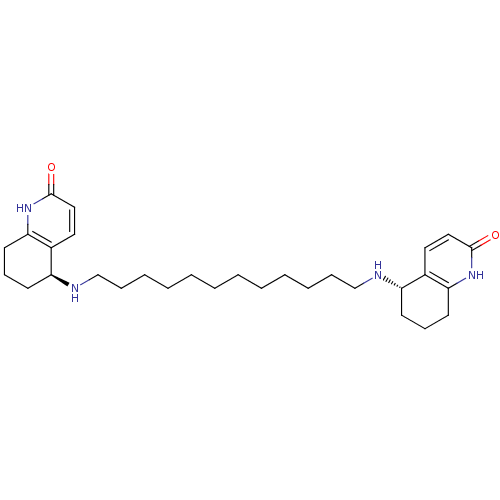 ((5S)-5-[(12-{[(5S)-2-oxo-1,2,5,6,7,8-hexahydroquin...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 4.5 | -11.3 | 16 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Weizmann Institute of Science | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at ... | J Am Chem Soc 125: 363-73 (2003) Article DOI: 10.1021/ja021111w BindingDB Entry DOI: 10.7270/Q26D5R69 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM10440 ((5S)-5-[(12-{[(5S)-2-oxo-1,2,5,6,7,8-hexahydroquin...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Inhibition of Torpedo californica AChE | J Med Chem 51: 3154-70 (2008) Article DOI: 10.1021/jm701253t BindingDB Entry DOI: 10.7270/Q22Z16D4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM10440 ((5S)-5-[(12-{[(5S)-2-oxo-1,2,5,6,7,8-hexahydroquin...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Inhibition of Torpedo californica AChE | J Med Chem 51: 3154-70 (2008) Article DOI: 10.1021/jm701253t BindingDB Entry DOI: 10.7270/Q22Z16D4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM10440 ((5S)-5-[(12-{[(5S)-2-oxo-1,2,5,6,7,8-hexahydroquin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 19.6 | -10.4 | 52 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Weizmann Institute of Science | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at ... | J Am Chem Soc 125: 363-73 (2003) Article DOI: 10.1021/ja021111w BindingDB Entry DOI: 10.7270/Q26D5R69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM10440 ((5S)-5-[(12-{[(5S)-2-oxo-1,2,5,6,7,8-hexahydroquin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Inhibition of rat AChE | J Med Chem 51: 3154-70 (2008) Article DOI: 10.1021/jm701253t BindingDB Entry DOI: 10.7270/Q22Z16D4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM10440 ((5S)-5-[(12-{[(5S)-2-oxo-1,2,5,6,7,8-hexahydroquin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Inhibition of rat AChE | J Med Chem 51: 3154-70 (2008) Article DOI: 10.1021/jm701253t BindingDB Entry DOI: 10.7270/Q22Z16D4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||