

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM105159 CHEMBL2152709::US8569281, 196
SMILES: Cc1[nH]nc2ccnc(OC3CCCC3)c12
InChI Key: InChIKey=HGALVNDKPMQZCT-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leucine-rich repeat serine/threonine-protein kinase 2 (LRRK2) (Homo sapiens (Human)) | BDBM105159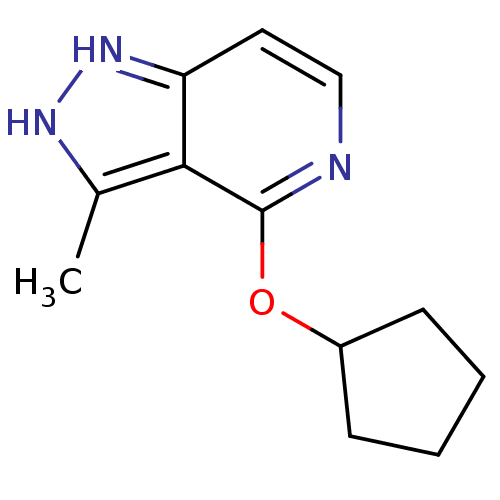 (CHEMBL2152709 | US8569281, 196) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 858 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity to LRRK2 | ACS Med Chem Lett 3: 701-702 (2012) Article DOI: 10.1021/ml300200p BindingDB Entry DOI: 10.7270/Q2514092 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (LRRK2) (Homo sapiens (Human)) | BDBM105159 (CHEMBL2152709 | US8569281, 196) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 858 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical Research Council Technology; Genentech, Inc. US Patent | Assay Description This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining Kiapp, IC50 or percent inhibition values. | US Patent US8569281 (2013) BindingDB Entry DOI: 10.7270/Q2DN43PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM105159 (CHEMBL2152709 | US8569281, 196) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
LifeArc Curated by ChEMBL | Assay Description Inhibition of recombinant human GST-tagged LRRK2 G2019S mutant catalytic domain (970 to 2527 residues) expressed in baculovirus expression system by ... | Bioorg Med Chem Lett 29: 668-673 (2019) Article DOI: 10.1016/j.bmcl.2018.11.058 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||