

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM11126 (2S)-2-amino-3-(4-phenylphenyl)-1-(pyrrolidin-1-yl)propan-1-one::4-phenylphenylalaninyl proline mimetic 13
SMILES: N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(=O)N1CCCC1
InChI Key: InChIKey=SXNUOZWYDHUUKD-SFHVURJKSA-N
Data: 1 KI
PDB links: 1 PDB ID contains this monomer as substructures. 1 PDB ID contains inhibitors having a similarity of 90% to this monomer.
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11126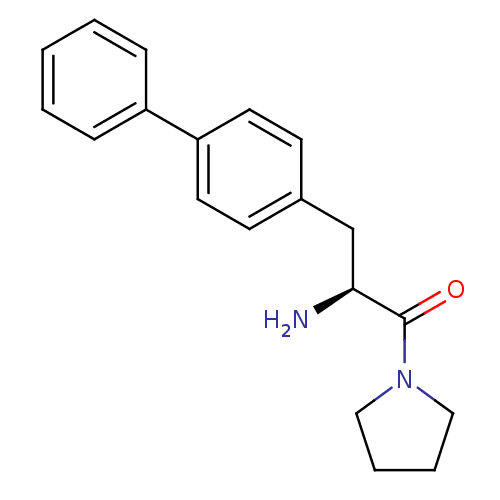 ((2S)-2-amino-3-(4-phenylphenyl)-1-(pyrrolidin-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.16E+3 | -8.09 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||