

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM11230 AG7088 analogue 2d::CHEMBL277716::N-[(benzyloxy)carbonyl]-L-valyl-N-((1S,2E)-4-ethoxy-4-oxo-1-{[(3S)-2-oxopyrrolidin-3-yl]methyl}but-2-enyl)-L-phenylalaninamide::TG-0205221 Analogue 2::cmdc.202100576, 5d::ethyl (2E,4S)-4-[(2S)-2-[(2S)-2-{[(benzyloxy)carbonyl]amino}-3-methylbutanamido]-3-phenylpropanamido]-5-[(3S)-2-oxopyrrolidin-3-yl]pent-2-enoate
SMILES: CCOC(=O)\C=C\[C@H](C[C@@H]1CCNC1=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)OCc1ccccc1)C(C)C
InChI Key: InChIKey=MQOSRSSZYUXZNH-XFFCECHOSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3C-like proteinase (3CL-PRO) (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM11230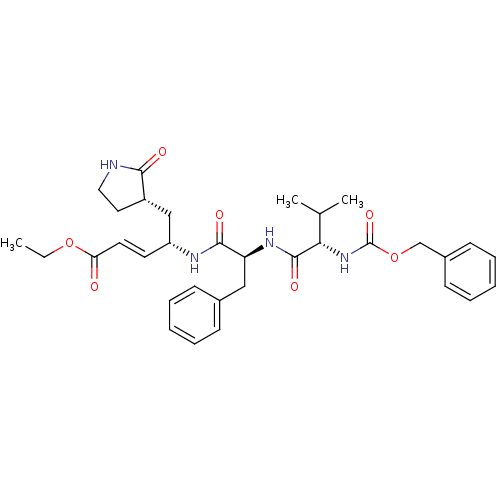 (AG7088 analogue 2d | CHEMBL277716 | N-[(benzyloxy)...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.26E+3 | n/a | 8.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Drug Development Centre | Assay Description Please point to the patents. | ChemMedChem (2021) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3C-like proteinase (3CL-PRO) (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM11230 (AG7088 analogue 2d | CHEMBL277716 | N-[(benzyloxy)...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.26E+3 | -7.70 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
TaiGen Biotechnology Co. | Assay Description The effects of compound on enzyme activity were measured by using peptide cleavage assay. Cleavage products were resolved and analyzed with a reverse... | J Med Chem 49: 4971-80 (2006) Article DOI: 10.1021/jm0603926 BindingDB Entry DOI: 10.7270/Q24B2ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3C-like proteinase (3CL-PRO) (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM11230 (AG7088 analogue 2d | CHEMBL277716 | N-[(benzyloxy)...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | 2.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3C-like proteinase (3CL-PRO) (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM11230 (AG7088 analogue 2d | CHEMBL277716 | N-[(benzyloxy)...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.50E+4 | n/a | n/a | n/a | n/a | 7.0 | 25 |
National Taiwan University | Assay Description The effects of compound on enzyme activity were measured by using a fluorogenic peptide cleavage assay. Enhanced fluorescence caused by cleavage of t... | Bioorg Med Chem 13: 5240-52 (2005) Article DOI: 10.1016/j.bmc.2005.05.065 BindingDB Entry DOI: 10.7270/Q2VT1Q9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human rhinovirus A protease (Human rhinovirus B) | BDBM11230 (AG7088 analogue 2d | CHEMBL277716 | N-[(benzyloxy)...) | MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 30 | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Increased percentage of formazan production in drug treated virus infected cells to equal 50% control drug free uninfected cells on serotype 14 | J Med Chem 42: 1213-24 (1999) Article DOI: 10.1021/jm9805384 BindingDB Entry DOI: 10.7270/Q2MW2G9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||