

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM112546 US8618103, I-42
SMILES: O=S(=O)(CC#N)N1CCC[C@@H](C1)Nc1ncccc1-c1cnc2[nH]ccc2n1
InChI Key: InChIKey=PLRGDPPEBDGPBL-ZDUSSCGKSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM112546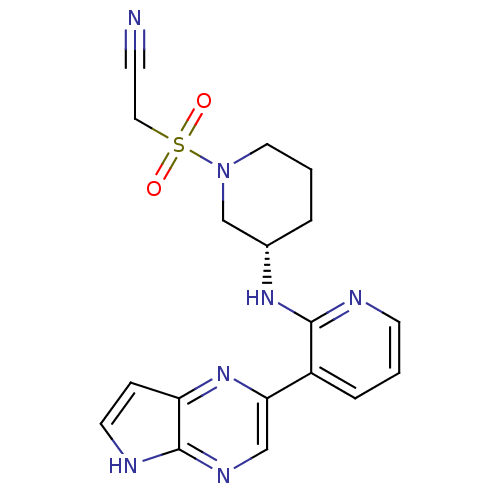 (US8618103, I-42) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.000748 | n/a | 0.00149 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description The kinase activity of all three JAK kinases is measured using a radioactive, end-point assay and with trace amounts of 33P-ATP. | US Patent US8618103 (2013) BindingDB Entry DOI: 10.7270/Q25H7DZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM112546 (US8618103, I-42) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.84E+3 | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of JAK2 in human PBMC expressing CD14 assessed as inhibition of GM-CSF-stimulated STAT5a phosphorylation after 30 mins by flow cytometric ... | Bioorg Med Chem Lett 24: 4969-75 (2014) Article DOI: 10.1016/j.bmcl.2014.09.031 BindingDB Entry DOI: 10.7270/Q25X2BPB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM112546 (US8618103, I-42) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of JAK2 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... | Bioorg Med Chem Lett 24: 4969-75 (2014) Article DOI: 10.1016/j.bmcl.2014.09.031 BindingDB Entry DOI: 10.7270/Q25X2BPB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM112546 (US8618103, I-42) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of JAK1 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... | Bioorg Med Chem Lett 24: 4969-75 (2014) Article DOI: 10.1016/j.bmcl.2014.09.031 BindingDB Entry DOI: 10.7270/Q25X2BPB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM112546 (US8618103, I-42) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0880 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description The kinase activity of all three JAK kinases is measured using a radioactive, end-point assay and with trace amounts of 33P-ATP. | US Patent US8618103 (2013) BindingDB Entry DOI: 10.7270/Q25H7DZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM112546 (US8618103, I-42) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of JAK3 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... | Bioorg Med Chem Lett 24: 4969-75 (2014) Article DOI: 10.1016/j.bmcl.2014.09.031 BindingDB Entry DOI: 10.7270/Q25X2BPB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||