

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM11310 4-(5-chlorothiophen-2-yl)-2-[(thiophene-2-sulfonyl)methyl]-1,3-thiazole hydrobromide::Compound 2 analog 21
SMILES: Clc1ccc(s1)-c1csc(CS(=O)(=O)c2cccs2)n1
InChI Key: InChIKey=RNWCXVNZHBPXSO-UHFFFAOYSA-N
Data: 1 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3C-like proteinase (3CL-PRO) (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM11310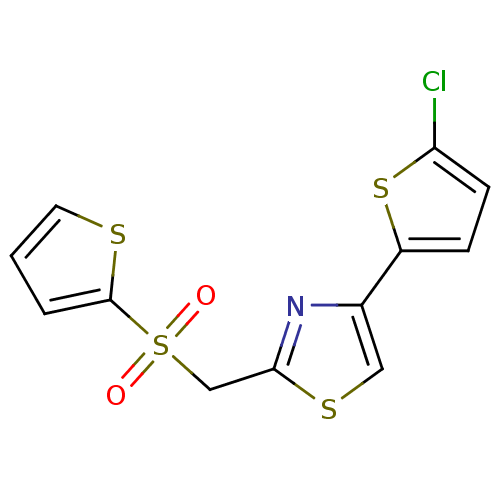 (4-(5-chlorothiophen-2-yl)-2-[(thiophene-2-sulfonyl...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Defense Medical Center | Assay Description The effects of compound on enzyme activity were measured by using a fluorogenic peptide cleavage assay. Enhanced fluorescence caused by cleavage of t... | J Med Chem 49: 5154-61 (2006) Article DOI: 10.1021/jm060207o BindingDB Entry DOI: 10.7270/Q2M906V0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||