

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM11637 (15S)-15-methyl-14-oxotetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadeca-2(7),3,5-trien-5-yl sulfamate::EMATE::estrone-3-O-sulfamate::sulfamate compound 4
SMILES: C[C@]12CCC3C(CCc4cc(OS(N)(=O)=O)ccc34)C1CCC2=O
InChI Key: InChIKey=RVKFQAJIXCZXQY-GUZDXLFXSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM11637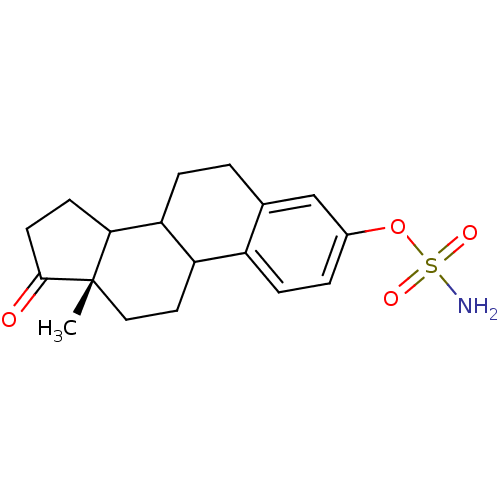 ((15S)-15-methyl-14-oxotetracyclo[8.7.0.0^{2,7}.0^{...) | PDB MMDB Reactome pathway KEGG B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 10 | -10.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Istituto di Biostrutture e Bioimmagini-CNR | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 48: 5721-7 (2005) Article DOI: 10.1021/jm050333c BindingDB Entry DOI: 10.7270/Q2ST7N2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM11637 ((15S)-15-methyl-14-oxotetracyclo[8.7.0.0^{2,7}.0^{...) | PDB MMDB NCI pathway Reactome pathway KEGG B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 30 | -10.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Istituto di Biostrutture e Bioimmagini-CNR | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 48: 5721-7 (2005) Article DOI: 10.1021/jm050333c BindingDB Entry DOI: 10.7270/Q2ST7N2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM11637 ((15S)-15-methyl-14-oxotetracyclo[8.7.0.0^{2,7}.0^{...) | PDB MMDB KEGG B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Maryamppound39s Hospital | Assay Description The extent of in vitro inhibition of sulfatase activities was assessed using intact monolayers of JEG-3 cells. Sulfatase activity was measured using ... | Biochem Biophys Res Commun 305: 909-14 (2003) Article DOI: 10.1016/s0006-291x(03)00865-9 BindingDB Entry DOI: 10.7270/Q2TD9VKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM11637 ((15S)-15-methyl-14-oxotetracyclo[8.7.0.0^{2,7}.0^{...) | PDB MMDB Reactome pathway KEGG B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | 7.8 | 20 |
St. Maryamppound39s Hospital | Assay Description The in vitro inhibition of carbonic anhydrase was assessed by a colorimetric assay. Carbonic anhydrase-catalysed hydrolysis of p-nitrophenyl acetate ... | Biochem Biophys Res Commun 305: 909-14 (2003) Article DOI: 10.1016/s0006-291x(03)00865-9 BindingDB Entry DOI: 10.7270/Q2TD9VKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM11637 ((15S)-15-methyl-14-oxotetracyclo[8.7.0.0^{2,7}.0^{...) | PDB MMDB Reactome pathway KEGG B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | 7.8 | 20 |
University of Bath | Assay Description The in vitro inhibition of carbonic anhydrase was assessed by a colorimetric assay. Carbonic anhydrase-catalysed hydrolysis of p-nitrophenyl acetate ... | Biochemistry 44: 6858-66 (2005) Article DOI: 10.1021/bi047692e BindingDB Entry DOI: 10.7270/Q2125QVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||