

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM119126 GS5
SMILES: C[C@@H](CC[C@H](C)NC(=O)[C@@H](NC(=O)N(C)Cc1csc(n1)C(C)C)C(C)C)NC(=O)OCc1cncs1
InChI Key: InChIKey=TYLOVNGZCFIJOG-WFXMLNOXSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM119126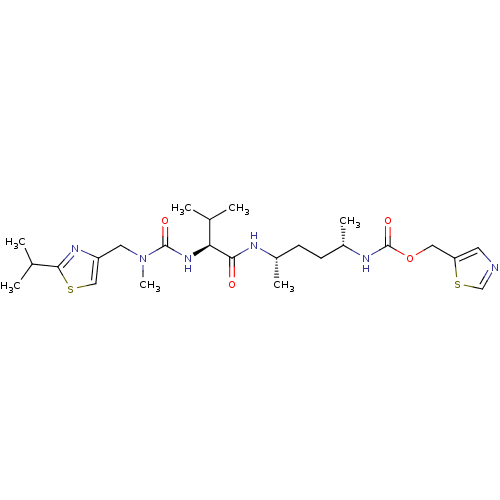 (GS5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine | Assay Description The inhibitory potency of compounds GS4-GS8 on the 7-benzyloxy-4-(trifluoromethyl)coumarin hydroxylase activity of CYP3A4 was evaluated according to ... | Biochemistry 52: 4474-81 (2013) Article DOI: 10.1021/bi4005396 BindingDB Entry DOI: 10.7270/Q2PZ57GJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM119126 (GS5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 7.30E+3 | n/a | n/a | n/a | 7.4 | n/a |
University of California at Irvine | Assay Description Ligand binidng to CYP3A4 was monitored in 50 mM phosphate (pH 7.4) containing 20% glycerol and 1 mM DTT. The protein was titrated with small aliquots... | Biochemistry 52: 4474-81 (2013) Article DOI: 10.1021/bi4005396 BindingDB Entry DOI: 10.7270/Q2PZ57GJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM119126 (GS5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 400 | n/a | n/a | n/a | 7.4 | n/a |
University of California at Irvine | Assay Description Ligand binidng to CYP3A4 was monitored in 50 mM phosphate (pH 7.4) containing 20% glycerol and 1 mM DTT. The protein was titrated with small aliquots... | Biochemistry 52: 4474-81 (2013) Article DOI: 10.1021/bi4005396 BindingDB Entry DOI: 10.7270/Q2PZ57GJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||