

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM11979 5-[(3-carboxy-4-hydroxyphenyl)methyl]-2-hydroxybenzoic acid::CHEMBL115145::MgrA inhibitor, 1::chemical diversity library compound 4
SMILES: OC(=O)c1cc(Cc2ccc(O)c(c2)C(O)=O)ccc1O
InChI Key: InChIKey=JWQFKVGACKJIAV-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anthrax Lethal Factor (LF) (Bacillus anthracis) | BDBM11979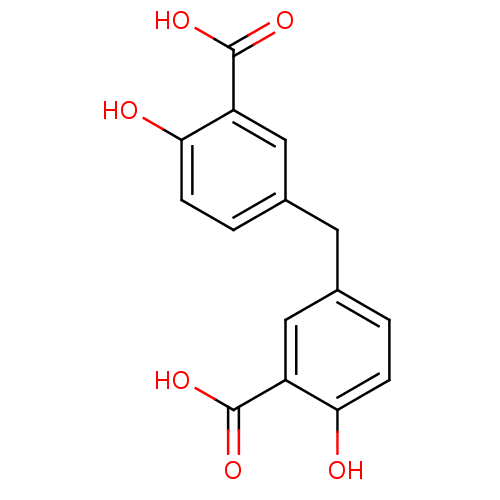 (5-[(3-carboxy-4-hydroxyphenyl)methyl]-2-hydroxyben...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.40E+3 | -7.71 | 2.90E+3 | n/a | n/a | n/a | n/a | 7.2 | 27 |
Montana State University | Assay Description For selected lead compounds from fluorescence-based high-throughput screening, Km and Vmax were calculated using the double-reciprocal Lineweaver-Bur... | J Med Chem 49: 5232-44 (2006) Article DOI: 10.1021/jm0605132 BindingDB Entry DOI: 10.7270/Q20C4T19 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 integrase (Human immunodeficiency virus 1) | BDBM11979 (5-[(3-carboxy-4-hydroxyphenyl)methyl]-2-hydroxyben...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.78E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibitory concentration of compound against strand transfer of HIV-1 integrase in experiment 2 | J Med Chem 40: 942-51 (1997) Article DOI: 10.1021/jm960759e BindingDB Entry DOI: 10.7270/Q2NK3FPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 integrase (Human immunodeficiency virus 1) | BDBM11979 (5-[(3-carboxy-4-hydroxyphenyl)methyl]-2-hydroxyben...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibitory concentration of compound against 3'-processing of HIV-1 integrase in experiment 1 | J Med Chem 40: 942-51 (1997) Article DOI: 10.1021/jm960759e BindingDB Entry DOI: 10.7270/Q2NK3FPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 integrase (Human immunodeficiency virus 1) | BDBM11979 (5-[(3-carboxy-4-hydroxyphenyl)methyl]-2-hydroxyben...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibitory concentration of compound against 3'-processing of HIV-1 integrase in experiment 2 | J Med Chem 40: 942-51 (1997) Article DOI: 10.1021/jm960759e BindingDB Entry DOI: 10.7270/Q2NK3FPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (DNMT1) (Homo sapiens (Human)) | BDBM11979 (5-[(3-carboxy-4-hydroxyphenyl)methyl]-2-hydroxyben...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Deutsches Krebsforschungszentrum Curated by ChEMBL | Assay Description Inhibition of human recombinant DNMT1 expressed in baculovirus-insect cell system by scintillation counting | Bioorg Med Chem 18: 822-9 (2010) Article DOI: 10.1016/j.bmc.2009.11.050 BindingDB Entry DOI: 10.7270/Q2CF9Q69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 3B (Homo sapiens (Human)) | BDBM11979 (5-[(3-carboxy-4-hydroxyphenyl)methyl]-2-hydroxyben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Deutsches Krebsforschungszentrum Curated by ChEMBL | Assay Description Inhibition of human recombinant DNMT3B expressed in baculovirus-insect cell system by scintillation counting | Bioorg Med Chem 18: 822-9 (2010) Article DOI: 10.1016/j.bmc.2009.11.050 BindingDB Entry DOI: 10.7270/Q2CF9Q69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 integrase (Human immunodeficiency virus 1) | BDBM11979 (5-[(3-carboxy-4-hydroxyphenyl)methyl]-2-hydroxyben...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibitory concentration of compound against strand transfer of HIV-1 integrase in experiment 1 | J Med Chem 40: 942-51 (1997) Article DOI: 10.1021/jm960759e BindingDB Entry DOI: 10.7270/Q2NK3FPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine phosphatase 1B (Homo sapiens (Human)) | BDBM11979 (5-[(3-carboxy-4-hydroxyphenyl)methyl]-2-hydroxyben...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.60E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Inhibition of PTP1B after 10 mins | Bioorg Med Chem Lett 17: 2760-4 (2007) Article DOI: 10.1016/j.bmcl.2007.02.069 BindingDB Entry DOI: 10.7270/Q2445N91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HTH-type transcriptional regulator mgrA (MgrA) (Staphylococcus aureus) | BDBM11979 (5-[(3-carboxy-4-hydroxyphenyl)methyl]-2-hydroxyben...) | PDB MMDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus Newman His6-tagged MgrA expressed in Escherichia coli BL21(DE3) using 5'-6-F-TAAACAACAAGTTGTCCAAA-3' as substrate... | J Med Chem 56: 1389-404 (2013) Article DOI: 10.1021/jm3014635 BindingDB Entry DOI: 10.7270/Q2BV7J0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus type 2) | BDBM11979 (5-[(3-carboxy-4-hydroxyphenyl)methyl]-2-hydroxyben...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel Curated by ChEMBL | Assay Description Inhibition of Dengue virus type 2 NS5 RNA methyltransferase SAM site | J Med Chem 53: 1483-95 (2010) Article DOI: 10.1021/jm900776m BindingDB Entry DOI: 10.7270/Q2ST7SNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus type 2) | BDBM11979 (5-[(3-carboxy-4-hydroxyphenyl)methyl]-2-hydroxyben...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel Curated by ChEMBL | Assay Description Inhibition of Dengue virus type 2 NS5 RNA methyltransferase SAM site with 0.1 % TX100 | J Med Chem 53: 1483-95 (2010) Article DOI: 10.1021/jm900776m BindingDB Entry DOI: 10.7270/Q2ST7SNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus type 2) | BDBM11979 (5-[(3-carboxy-4-hydroxyphenyl)methyl]-2-hydroxyben...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel Curated by ChEMBL | Assay Description Inhibition of Dengue virus type 2 NS5 RNA methyltransferase by spin-down assay | J Med Chem 53: 1483-95 (2010) Article DOI: 10.1021/jm900776m BindingDB Entry DOI: 10.7270/Q2ST7SNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus type 2) | BDBM11979 (5-[(3-carboxy-4-hydroxyphenyl)methyl]-2-hydroxyben...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel Curated by ChEMBL | Assay Description Inhibition of Dengue virus type 2 NS5 RNA methyltransferase at 8 nM enzyme concentration | J Med Chem 53: 1483-95 (2010) Article DOI: 10.1021/jm900776m BindingDB Entry DOI: 10.7270/Q2ST7SNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus type 2) | BDBM11979 (5-[(3-carboxy-4-hydroxyphenyl)methyl]-2-hydroxyben...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel Curated by ChEMBL | Assay Description Inhibition of Dengue virus type 2 NS5 RNA methyltransferase at 80 nM enzyme concentration | J Med Chem 53: 1483-95 (2010) Article DOI: 10.1021/jm900776m BindingDB Entry DOI: 10.7270/Q2ST7SNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 3-like (Homo sapiens (Human)) | BDBM11979 (5-[(3-carboxy-4-hydroxyphenyl)methyl]-2-hydroxyben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ du Qu£bec £ Montr£al Curated by ChEMBL | Assay Description Inhibition of DNMT3A/3L (unknown origin) using poly(2'-deoxyinosinic-2'-deoxycytidylic acid) as substrate after 1 hr by scintillation proximity assay | Bioorg Med Chem Lett 29: 826-831 (2019) Article DOI: 10.1016/j.bmcl.2019.01.022 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNMT3B-DNMT3L complex (Homo sapiens (Human)) | BDBM11979 (5-[(3-carboxy-4-hydroxyphenyl)methyl]-2-hydroxyben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ du Qu£bec £ Montr£al Curated by ChEMBL | Assay Description Inhibition of DNMT3B/3L (unknown origin) using poly(2'-deoxyinosinic-2'-deoxycytidylic acid) as substrate after 1 hr by scintillation proximity assay | Bioorg Med Chem Lett 29: 826-831 (2019) Article DOI: 10.1016/j.bmcl.2019.01.022 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM11979 (5-[(3-carboxy-4-hydroxyphenyl)methyl]-2-hydroxyben...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ du Qu£bec £ Montr£al Curated by ChEMBL | Assay Description Inhibition of DNMT1 (unknown origin) using biotinylated substrate using [3H]-SAM after 1 hr by scintillation proximity assay | Bioorg Med Chem Lett 29: 826-831 (2019) Article DOI: 10.1016/j.bmcl.2019.01.022 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HTH-type transcriptional regulator mgrA (MgrA) (Staphylococcus aureus) | BDBM11979 (5-[(3-carboxy-4-hydroxyphenyl)methyl]-2-hydroxyben...) | PDB MMDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | 7.4 | 7.7 |
The University of Chicago | Assay Description A fluorescence anisotropy (FA)-based biochemical assay that monitors MgrA-DNA binding. To determine the binding affinity of MgrA to the labeled DNA,... | Chem Biol 18: 1032-41 (2011) Article DOI: 10.1016/j.chembiol.2011.05.014 BindingDB Entry DOI: 10.7270/Q2CN72DF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||