

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM121388 US8722890, 3
SMILES: C[C@H](O)Cn1cc(cn1)-c1cnc(N)c2c(csc12)-c1ccc(NC(=O)Nc2cccc(C)c2)cc1
InChI Key: InChIKey=VKBFHTIUXUCYRQ-KRWDZBQOSA-N
Data: 9 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM121388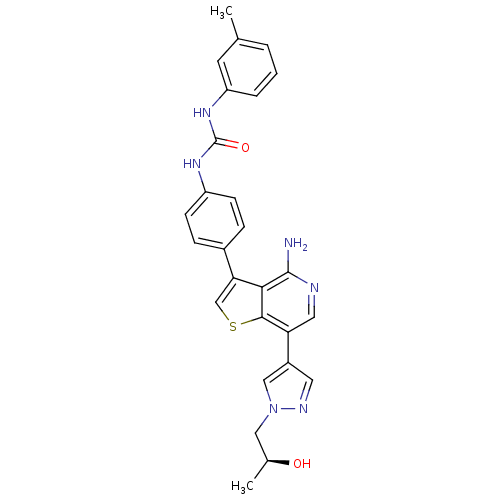 (US8722890, 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | 7.1 | 25 |
AbbVie Inc. US Patent | Assay Description To determine the activity of the various kinases, a homogenous time-resolved fluorescence (HTRF) in vitro kinase assay was used. (Mathis, G., HTRF(R)... | US Patent US8722890 (2014) BindingDB Entry DOI: 10.7270/Q21Z4327 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 1 (Homo sapiens (Human)) | BDBM121388 (US8722890, 3) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.04 | n/a | n/a | n/a | n/a | 7.1 | 25 |
AbbVie Inc. US Patent | Assay Description To determine the activity of the various kinases, a homogenous time-resolved fluorescence (HTRF) in vitro kinase assay was used. (Mathis, G., HTRF(R)... | US Patent US8722890 (2014) BindingDB Entry DOI: 10.7270/Q21Z4327 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony stimulating factor receptor (Homo sapiens (Human)) | BDBM121388 (US8722890, 3) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.62 | n/a | n/a | n/a | n/a | 7.1 | 25 |
AbbVie Inc. US Patent | Assay Description To determine the activity of the various kinases, a homogenous time-resolved fluorescence (HTRF) in vitro kinase assay was used. (Mathis, G., HTRF(R)... | US Patent US8722890 (2014) BindingDB Entry DOI: 10.7270/Q21Z4327 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM121388 (US8722890, 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.18 | n/a | n/a | n/a | n/a | 7.1 | 25 |
AbbVie Inc. US Patent | Assay Description To determine the activity of the various kinases, a homogenous time-resolved fluorescence (HTRF) in vitro kinase assay was used. (Mathis, G., HTRF(R)... | US Patent US8722890 (2014) BindingDB Entry DOI: 10.7270/Q21Z4327 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM121388 (US8722890, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
AbbVie Inc. US Patent | Assay Description Assays (200 μL final volume) were carried out in NUNC polypropylene deep well plates in 50 mM potassium phosphate buffer, pH 7.4, using a microt... | US Patent US8722890 (2014) BindingDB Entry DOI: 10.7270/Q21Z4327 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM121388 (US8722890, 3) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 11.2 | n/a | n/a | n/a | n/a | 7.4 | n/a |
AbbVie Inc. US Patent | Assay Description To determine Aurora B activity of representative compounds of the invention, Active Aurora B enzyme (recombinant residues 1-344) and INCENP (recombin... | US Patent US8722890 (2014) BindingDB Entry DOI: 10.7270/Q21Z4327 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM121388 (US8722890, 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 248 | n/a | n/a | n/a | n/a | 7.4 | n/a |
AbbVie Inc. US Patent | Assay Description To determine Aurora A and C activity of representative compounds of the invention, Active Aurora A or C enzyme was incubated in wells of a 384 well p... | US Patent US8722890 (2014) BindingDB Entry DOI: 10.7270/Q21Z4327 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM121388 (US8722890, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
AbbVie Inc. US Patent | Assay Description Assays (200 μL final volume) were carried out in NUNC polypropylene deep well plates in 50 mM potassium phosphate buffer, pH 7.4, using a microt... | US Patent US8722890 (2014) BindingDB Entry DOI: 10.7270/Q21Z4327 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast/stem cell growth factor receptor Kit (Homo sapiens (Human)) | BDBM121388 (US8722890, 3) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 27.1 | n/a | n/a | n/a | n/a | 7.1 | 25 |
AbbVie Inc. US Patent | Assay Description To determine the activity of the various kinases, a homogenous time-resolved fluorescence (HTRF) in vitro kinase assay was used. (Mathis, G., HTRF(R)... | US Patent US8722890 (2014) BindingDB Entry DOI: 10.7270/Q21Z4327 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||