

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM12192 (2S)-2-amino-2-cyclohexyl-1-(2,3-dihydro-1H-isoindol-2-yl)ethan-1-one::isoquinoline derivative 15
SMILES: N[C@@H](C1CCCCC1)C(=O)N1Cc2ccccc2C1
InChI Key: InChIKey=SUSVSQMWKFICCC-HNNXBMFYSA-N
Data: 3 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dipeptidyl peptidase VIII (Homo sapiens (Human)) | BDBM12192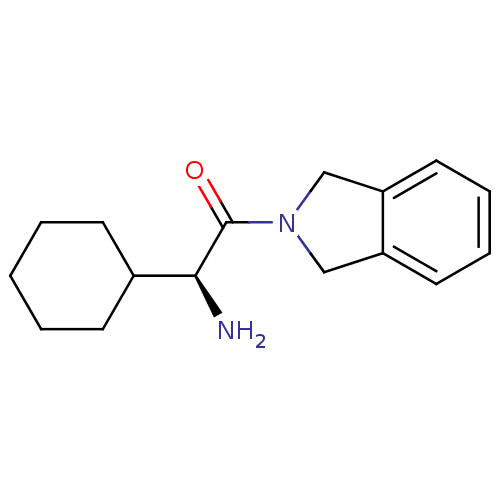 ((2S)-2-amino-2-cyclohexyl-1-(2,3-dihydro-1H-isoind...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | 8.0 | 37 |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 15: 687-91 (2005) Article DOI: 10.1016/j.bmcl.2004.11.023 BindingDB Entry DOI: 10.7270/Q2XG9PDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (DPP II) (Homo sapiens (Human)) | BDBM12192 ((2S)-2-amino-2-cyclohexyl-1-(2,3-dihydro-1H-isoind...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 15: 687-91 (2005) Article DOI: 10.1016/j.bmcl.2004.11.023 BindingDB Entry DOI: 10.7270/Q2XG9PDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM12192 ((2S)-2-amino-2-cyclohexyl-1-(2,3-dihydro-1H-isoind...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | Bioorg Med Chem Lett 15: 687-91 (2005) Article DOI: 10.1016/j.bmcl.2004.11.023 BindingDB Entry DOI: 10.7270/Q2XG9PDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||