

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM12659 1-(3-Aminobenzisoxazol-5-yl)-3-trifluoromethyl-5-[(2-methylsulfonyl-3-fluoro[1,1]biphen-4-yl)aminocarbonyl]-pyrazole, Trifluoroacetic Acid::1-(3-amino-1,2-benzoxazol-5-yl)-N-[2-fluoro-4-(2-methanesulfonylphenyl)phenyl]-3-(trifluoromethyl)-1H-pyrazole-5-carboxamide; 2,2,2-trifluoroacetic acid::CHEMBL554926::Razaxaban Analogue::aminobenzisoxazole 38
SMILES: CS(=O)(=O)c1ccccc1-c1ccc(NC(=O)c2cc(nn2-c2ccc3onc(N)c3c2)C(F)(F)F)c(F)c1
InChI Key: InChIKey=GTYYDEJOAHEDLZ-UHFFFAOYSA-N
Data: 8 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor X (Homo sapiens (Human)) | BDBM12659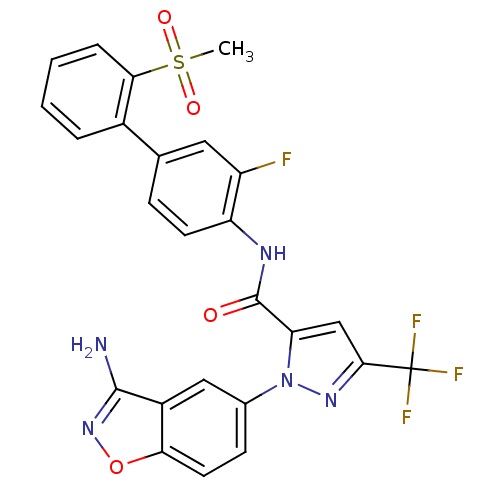 (1-(3-Aminobenzisoxazol-5-yl)-3-trifluoromethyl-5-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0900 | -13.6 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | J Med Chem 48: 1729-44 (2005) Article DOI: 10.1021/jm0497949 BindingDB Entry DOI: 10.7270/Q2BK19K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12659 (1-(3-Aminobenzisoxazol-5-yl)-3-trifluoromethyl-5-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of human factor 10a | Bioorg Med Chem Lett 17: 6481-8 (2007) Article DOI: 10.1016/j.bmcl.2007.09.091 BindingDB Entry DOI: 10.7270/Q2GX4B9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM12659 (1-(3-Aminobenzisoxazol-5-yl)-3-trifluoromethyl-5-[...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | J Med Chem 48: 1729-44 (2005) Article DOI: 10.1021/jm0497949 BindingDB Entry DOI: 10.7270/Q2BK19K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM12659 (1-(3-Aminobenzisoxazol-5-yl)-3-trifluoromethyl-5-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | J Med Chem 48: 1729-44 (2005) Article DOI: 10.1021/jm0497949 BindingDB Entry DOI: 10.7270/Q2BK19K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypsin (Bos taurus (bovine)) | BDBM12659 (1-(3-Aminobenzisoxazol-5-yl)-3-trifluoromethyl-5-[...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | J Med Chem 48: 1729-44 (2005) Article DOI: 10.1021/jm0497949 BindingDB Entry DOI: 10.7270/Q2BK19K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM12659 (1-(3-Aminobenzisoxazol-5-yl)-3-trifluoromethyl-5-[...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | J Med Chem 48: 1729-44 (2005) Article DOI: 10.1021/jm0497949 BindingDB Entry DOI: 10.7270/Q2BK19K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM12659 (1-(3-Aminobenzisoxazol-5-yl)-3-trifluoromethyl-5-[...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of human thrombin | Bioorg Med Chem Lett 17: 6481-8 (2007) Article DOI: 10.1016/j.bmcl.2007.09.091 BindingDB Entry DOI: 10.7270/Q2GX4B9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM12659 (1-(3-Aminobenzisoxazol-5-yl)-3-trifluoromethyl-5-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | J Med Chem 48: 1729-44 (2005) Article DOI: 10.1021/jm0497949 BindingDB Entry DOI: 10.7270/Q2BK19K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||