

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM12916 CHEMBL386542::N-(Prop-2-ynyl)-4-sulfamoylbenzamide::N-(prop-2-yn-1-yl)-4-sulfamoylbenzamide::N-propynyl amide derivative 2
SMILES: NS(=O)(=O)c1ccc(cc1)C(=O)NCC#C
InChI Key: InChIKey=KRDXLVUMSYUYOP-UHFFFAOYSA-N
Data: 5 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM12916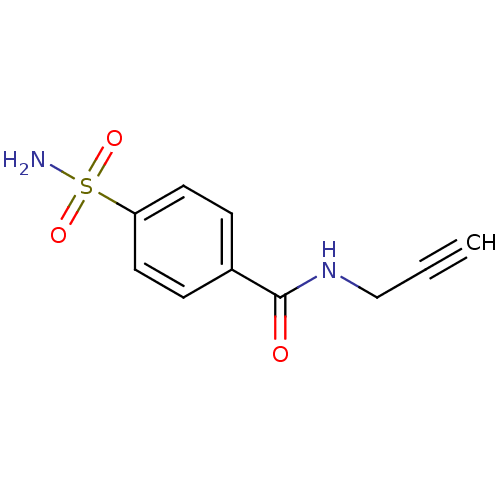 (CHEMBL386542 | N-(Prop-2-ynyl)-4-sulfamoylbenzamid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University Curated by ChEMBL | Assay Description Inhibition of human recombinant CA12 by CO2 hydration assay | Bioorg Med Chem Lett 17: 987-92 (2007) Article DOI: 10.1016/j.bmcl.2006.11.046 BindingDB Entry DOI: 10.7270/Q2JD4XMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic Anhydrase XIV (Homo sapiens (Human)) | BDBM12916 (CHEMBL386542 | N-(Prop-2-ynyl)-4-sulfamoylbenzamid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 11.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University Curated by ChEMBL | Assay Description Inhibition of human recombinant CA14 by CO2 hydration assay | Bioorg Med Chem Lett 17: 987-92 (2007) Article DOI: 10.1016/j.bmcl.2006.11.046 BindingDB Entry DOI: 10.7270/Q2JD4XMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM12916 (CHEMBL386542 | N-(Prop-2-ynyl)-4-sulfamoylbenzamid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 49: 6539-48 (2006) Article DOI: 10.1021/jm060967z BindingDB Entry DOI: 10.7270/Q2X928HW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM12916 (CHEMBL386542 | N-(Prop-2-ynyl)-4-sulfamoylbenzamid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 113 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 49: 6539-48 (2006) Article DOI: 10.1021/jm060967z BindingDB Entry DOI: 10.7270/Q2X928HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM12916 (CHEMBL386542 | N-(Prop-2-ynyl)-4-sulfamoylbenzamid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.10E+3 | -7.11 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Griffith University | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 49: 6539-48 (2006) Article DOI: 10.1021/jm060967z BindingDB Entry DOI: 10.7270/Q2X928HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||