

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM130909 US10683289, Example Staurosporine::US10927120, Compound staurosporine::US8822500, Stauro- sporine::US9920060, Staurosporine
SMILES: CNC1CC2OC(C)(C1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13
InChI Key: InChIKey=HKSZLNNOFSGOKW-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine/threonine-protein kinase receptor R3 (Homo sapiens (Human)) | BDBM130909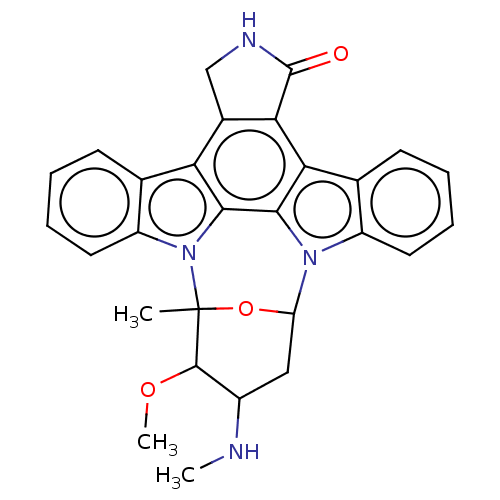 (US10683289, Example Staurosporine | US10927120, Co...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 1.36 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Chembridge Corporation US Patent | Assay Description Compounds to be tested were dissolved in 100% DMSO in a range of concentrations from 10-8 to 10-3 M including negative control (DMSO), then diluted w... | US Patent US8822500 (2014) BindingDB Entry DOI: 10.7270/Q2319TKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor I receptor (Homo sapiens (Human)) | BDBM130909 (US10683289, Example Staurosporine | US10927120, Co...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 71.5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Chembridge Corporation US Patent | Assay Description Compounds to be tested were dissolved in 100% DMSO in a range of concentrations from 10-8 to 10-3 M including negative control (DMSO), then diluted w... | US Patent US8822500 (2014) BindingDB Entry DOI: 10.7270/Q2319TKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM130909 (US10683289, Example Staurosporine | US10927120, Co...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 36.4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Chembridge Corporation US Patent | Assay Description Compounds to be tested were dissolved in 100% DMSO in a range of concentrations from 10-8 to 10-3 M including negative control (DMSO), then diluted w... | US Patent US8822500 (2014) BindingDB Entry DOI: 10.7270/Q2319TKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM130909 (US10683289, Example Staurosporine | US10927120, Co...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 1.41 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Chembridge Corporation US Patent | Assay Description Compounds to be tested were dissolved in 100% DMSO in a range of concentrations from 10-8 to 10-3 M including negative control (DMSO), then diluted w... | US Patent US8822500 (2014) BindingDB Entry DOI: 10.7270/Q2319TKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| BDNF/NT-3 growth factors receptor (Homo sapiens (Human)) | BDBM130909 (US10683289, Example Staurosporine | US10927120, Co...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 1.18 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Chembridge Corporation US Patent | Assay Description Compounds to be tested were dissolved in 100% DMSO in a range of concentrations from 10-8 to 10-3 M including negative control (DMSO), then diluted w... | US Patent US8822500 (2014) BindingDB Entry DOI: 10.7270/Q2319TKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NT-3 growth factor receptor (Homo sapiens (Human)) | BDBM130909 (US10683289, Example Staurosporine | US10927120, Co...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 0.525 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Chembridge Corporation US Patent | Assay Description Compounds to be tested were dissolved in 100% DMSO in a range of concentrations from 10-8 to 10-3 M including negative control (DMSO), then diluted w... | US Patent US8822500 (2014) BindingDB Entry DOI: 10.7270/Q2319TKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-Dependent Kinase 4 (CDK4) (Homo sapiens (Human)) | BDBM130909 (US10683289, Example Staurosporine | US10927120, Co...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 37.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GI Therapeutics, Inc. US Patent | Assay Description For the measurement of CDK2/cyclinE activity, enzyme (0.22 nM) was incubated with 100 mM ATP and the phosphoacceptor substrate peptide (1 mM) for one... | US Patent US10927120 (2021) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM130909 (US10683289, Example Staurosporine | US10927120, Co...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 2.19 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Chembridge Corporation US Patent | Assay Description Compounds to be tested were dissolved in 100% DMSO in a range of concentrations from 10-8 to 10-3 M including negative control (DMSO), then diluted w... | US Patent US8822500 (2014) BindingDB Entry DOI: 10.7270/Q2319TKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM130909 (US10683289, Example Staurosporine | US10927120, Co...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 107 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Chembridge Corporation US Patent | Assay Description Compounds to be tested were dissolved in 100% DMSO in a range of concentrations from 10-8 to 10-3 M including negative control (DMSO), then diluted w... | US Patent US8822500 (2014) BindingDB Entry DOI: 10.7270/Q2319TKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM130909 (US10683289, Example Staurosporine | US10927120, Co...) | PDB MMDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Paris 7-Denis Diderot | Assay Description For the inhibitory activity measurement of each compound, the compound of the present invention or staurosporine was first serially diluted with dime... | Bioorg Med Chem 16: 1242-53 (2008) BindingDB Entry DOI: 10.7270/Q2BR8VHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 5 (Homo sapiens (Human)) | BDBM130909 (US10683289, Example Staurosporine | US10927120, Co...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Enanta Pharmaceuticals, Inc. US Patent | Assay Description ASK1 was purchased from Thermofisher (Catalogue # PV4011), ATP was purchased from Sigma (Catalogue # A7699), HTRF® KinEASE™ Assay System was obtained... | US Patent US10683289 (2020) | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| CDK2/CycE (Homo sapiens (Human)) | BDBM130909 (US10683289, Example Staurosporine | US10927120, Co...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 3.93 | n/a | n/a | n/a | n/a | n/a | n/a |
GI Therapeutics, Inc. US Patent | Assay Description For the measurement of CDK2/cyclinE activity, enzyme (0.22 nM) was incubated with 100 mM ATP and the phosphoacceptor substrate peptide (1 mM) for one... | US Patent US10927120 (2021) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase ROS (Homo sapiens (Human)) | BDBM130909 (US10683289, Example Staurosporine | US10927120, Co...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 0.248 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Chembridge Corporation US Patent | Assay Description Compounds to be tested were dissolved in 100% DMSO in a range of concentrations from 10-8 to 10-3 M including negative control (DMSO), then diluted w... | US Patent US8822500 (2014) BindingDB Entry DOI: 10.7270/Q2319TKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||