

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM13137 (2R)-N-hydroxy-2-[(4-methoxybenzene)(pyridin-3-ylmethyl)sulfonamido]-4-methylpentanamide::CGS 27023A Analog 69
SMILES: COc1ccc(cc1)S(=O)(=O)N(Cc1cccnc1)[C@H](CC(C)C)C(=O)NO
InChI Key: InChIKey=QXKPLUFQYAQXMP-GOSISDBHSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stromelysin-1 (Homo sapiens (Human)) | BDBM13137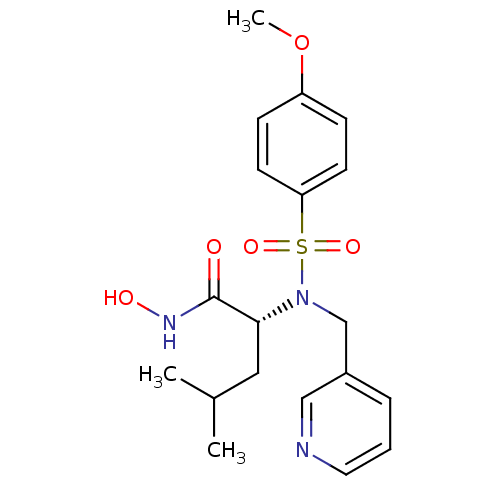 ((2R)-N-hydroxy-2-[(4-methoxybenzene)(pyridin-3-ylm...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Stromelysin inhibitory activity is based on the hydrolysis of substance P by recombinant human stromelysin to generate a fragment, substance P 7-11, ... | J Med Chem 40: 2525-32 (1997) Article DOI: 10.1021/jm960871c BindingDB Entry DOI: 10.7270/Q2MW2FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||