

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM13304 4-hydroxy-3-[((3S)-2-oxo-3-{[(5-pyridin-3-ylthien-2-yl)sulfonyl]amino}pyrrolidin-1-yl)methyl]benzenecarboximidamide::4-hydroxy-3-{[(3S)-2-oxo-3-{[5-(pyridin-3-yl)thiophene-2-]sulfonamido}pyrrolidin-1-yl]methyl}benzene-1-carboximidamide::RPR130737::Sulfonamidopyrrolidinone 20b
SMILES: NC(=N)c1ccc(O)c(CN2CC[C@H](NS(=O)(=O)c3ccc(s3)-c3cccnc3)C2=O)c1
InChI Key: InChIKey=MKXLWSHXRLAPHM-INIZCTEOSA-N
Data: 2 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor X (Homo sapiens (Human)) | BDBM13304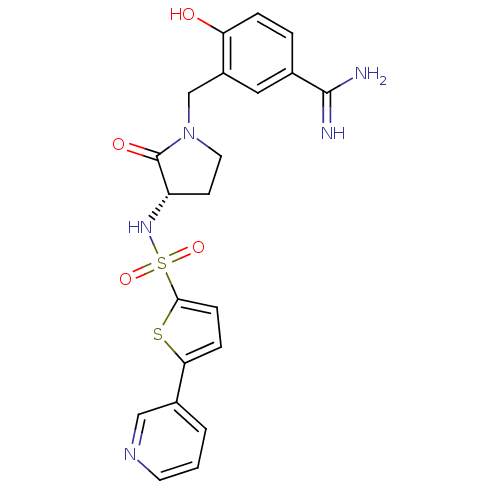 (4-hydroxy-3-[((3S)-2-oxo-3-{[(5-pyridin-3-ylthien-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 42: 3572-87 (1999) Article DOI: 10.1021/jm990041+ BindingDB Entry DOI: 10.7270/Q2KW5D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypsin (Bos taurus (bovine)) | BDBM13304 (4-hydroxy-3-[((3S)-2-oxo-3-{[(5-pyridin-3-ylthien-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 42: 3572-87 (1999) Article DOI: 10.1021/jm990041+ BindingDB Entry DOI: 10.7270/Q2KW5D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||