

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM13305 4-Amino-3-[3-(S)-(5-isoxazol-3-ylthiophene-2-ylsulfonylamino)-2-oxopyrrolidin-1-ylmethyl]benzamidine::4-amino-3-{[(3S)-3-{[5-(1,2-oxazol-3-yl)thiophene-2-]sulfonamido}-2-oxopyrrolidin-1-yl]methyl}benzene-1-carboximidamide::Sulfonamidopyrrolidinone 4d
SMILES: NC(=N)c1ccc(N)c(CN2CC[C@H](NS(=O)(=O)c3ccc(s3)-c3ccon3)C2=O)c1
InChI Key: InChIKey=JZYCCVRLQKHTCR-HNNXBMFYSA-N
Data: 2 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor X (Homo sapiens (Human)) | BDBM13305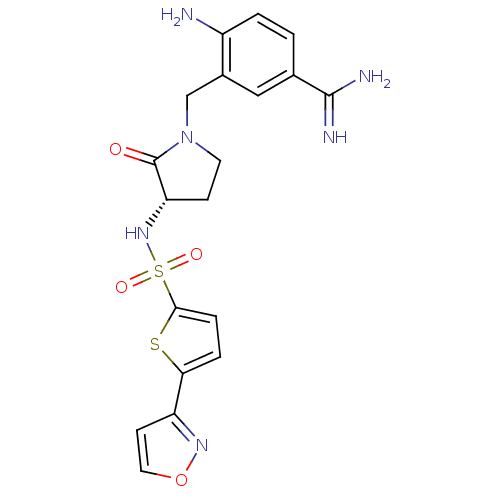 (4-Amino-3-[3-(S)-(5-isoxazol-3-ylthiophene-2-ylsul...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 42: 3572-87 (1999) Article DOI: 10.1021/jm990041+ BindingDB Entry DOI: 10.7270/Q2KW5D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypsin (Bos taurus (bovine)) | BDBM13305 (4-Amino-3-[3-(S)-(5-isoxazol-3-ylthiophene-2-ylsul...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 42: 3572-87 (1999) Article DOI: 10.1021/jm990041+ BindingDB Entry DOI: 10.7270/Q2KW5D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||