

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM13469 ({4-[(2S)-2-carbamoyl-2-[(2S)-2-(1-{4-[difluoro(phosphono)methyl]phenyl}acetamido)-3-phenylpropanamido]ethyl]phenyl}difluoromethyl)phosphonic acid::Difluoromethylphosphonic acid (DFMP) deriv. 1::[[4-[[(1S)-1-[[(1S)-1-aminocarbonyl-2-[4-(difluoro-phosphono-methyl)phenyl]ethyl]carbamoyl]-2-phenyl-ethyl]carbamoylmethyl]phenyl]-difluoro-methyl]phosphonic acid
SMILES: NC(=O)[C@H](Cc1ccc(cc1)C(F)(F)P(O)(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)Cc1ccc(cc1)C(F)(F)P(O)(O)=O
InChI Key: InChIKey=LGZYIQKUXWAUAL-GOTSBHOMSA-N
PDB links: 1 PDB ID matches this monomer. 1 PDB ID contains inhibitors having a similarity of 90% to this monomer.
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein-tyrosine phosphatase 1B (Homo sapiens (Human)) | BDBM13469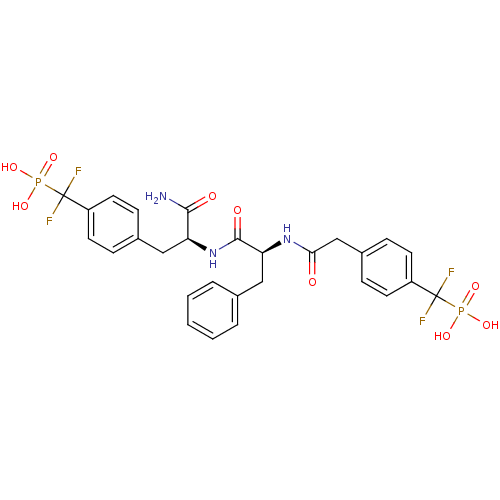 (({4-[(2S)-2-carbamoyl-2-[(2S)-2-(1-{4-[difluoro(ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Qilu University of Technology Curated by ChEMBL | Assay Description Inhibition of human PTP1B catalytic domain expressed in Escherichia coli assessed as pNPP hydrolysis measured every 30 secs for 15 mins | Bioorg Med Chem 23: 4891-8 (2015) Article DOI: 10.1016/j.bmc.2015.05.032 BindingDB Entry DOI: 10.7270/Q28917N0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protein-Tyrosine Phosphatase 1B (PTP1B) (Homo sapiens (Human)) | BDBM13469 (({4-[(2S)-2-carbamoyl-2-[(2S)-2-(1-{4-[difluoro(ph...) | PDB MMDB B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Incyte Corporation | Assay Description The activity of PTP1B enzyme was assayed with 4-nitrophenyl phosphate (pNPP) as substrate. Rate of formation of the phenolate ion was monitored at 41... | J Biol Chem 281: 38013-21 (2006) Article DOI: 10.1074/jbc.M607913200 BindingDB Entry DOI: 10.7270/Q2JW8C4X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||