

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM13618 3-Oxybenzamide 4::3-[2-(5-chloropyridin-2-yl)ethoxy]-4-fluoro-N-{[1-(pyridin-4-yl)piperidin-4-yl]methyl}benzamide
SMILES: Fc1ccc(cc1OCCc1ccc(Cl)cn1)C(=O)NCC1CCN(CC1)c1ccncc1
InChI Key: InChIKey=LTHCKUVKEGEEGL-UHFFFAOYSA-N
Data: 4 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor X (Homo sapiens (Human)) | BDBM13618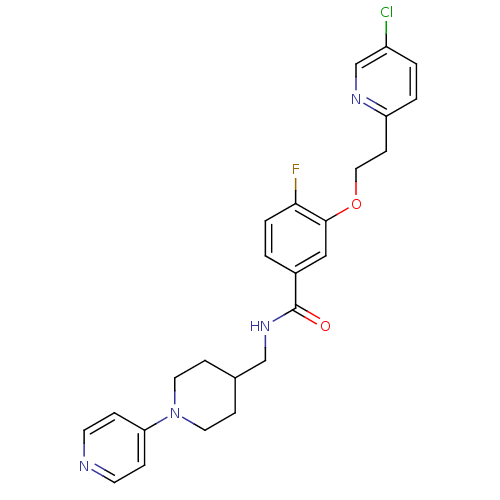 (3-Oxybenzamide 4 | 3-[2-(5-chloropyridin-2-yl)etho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 37 | -10.1 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 3290-312 (2005) Article DOI: 10.1021/jm049187l BindingDB Entry DOI: 10.7270/Q28C9THZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13618 (3-Oxybenzamide 4 | 3-[2-(5-chloropyridin-2-yl)etho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH Curated by ChEMBL | Assay Description Inhibitory activity against human Coagulation factor X | Bioorg Med Chem Lett 14: 2801-5 (2004) Article DOI: 10.1016/j.bmcl.2004.03.059 BindingDB Entry DOI: 10.7270/Q2XS5WW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13618 (3-Oxybenzamide 4 | 3-[2-(5-chloropyridin-2-yl)etho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 39.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universita£t Curated by ChEMBL | Assay Description Inhibition of human factor 10a | J Med Chem 54: 2944-51 (2011) Article DOI: 10.1021/jm200026b BindingDB Entry DOI: 10.7270/Q2ZG6TGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM13618 (3-Oxybenzamide 4 | 3-[2-(5-chloropyridin-2-yl)etho...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.00E+4 | -6.82 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human thrombin was determined by using the chromogenic substrates S-2366. The hydrolysis rates of chromoge... | J Med Chem 48: 3290-312 (2005) Article DOI: 10.1021/jm049187l BindingDB Entry DOI: 10.7270/Q28C9THZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||