

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM13987 1:1 racemic mixture::2-{4-[2-acetamido-2-({4-[3-hydroxy-2-(methoxycarbonyl)phenoxy]butyl}carbamoyl)ethyl]-2-hydroxyphenoxy}acetic acid::phosphotyrosyl mimetic 20
SMILES: COC(=O)c1c(O)cccc1OCCCCNC(=O)C(Cc1ccc(OCC(O)=O)c(O)c1)NC(C)=O
InChI Key: InChIKey=QEANZBIJPCAREV-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein-tyrosine phosphatase 1B (Homo sapiens (Human)) | BDBM13987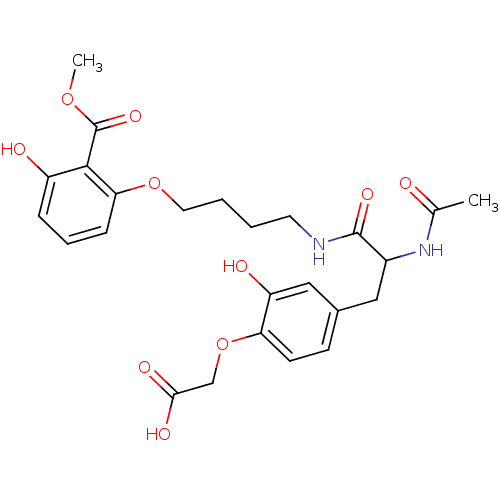 (1:1 racemic mixture | 2-{4-[2-acetamido-2-({4-[3-h...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 7.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of PTP1B | J Med Chem 52: 3159-65 (2009) Article DOI: 10.1021/jm801444x BindingDB Entry DOI: 10.7270/Q2FF3TM8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protein-Tyrosine Phosphatase 1B (PTP1B) (Homo sapiens (Human)) | BDBM13987 (1:1 racemic mixture | 2-{4-[2-acetamido-2-({4-[3-h...) | PDB MMDB B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 9.00E+3 | -6.81 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 13: 3947-50 (2003) Article DOI: 10.1016/j.bmcl.2003.08.064 BindingDB Entry DOI: 10.7270/Q22R3PXB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM13987 (1:1 racemic mixture | 2-{4-[2-acetamido-2-({4-[3-h...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 1.82E+5 | -5.05 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 13: 3947-50 (2003) Article DOI: 10.1016/j.bmcl.2003.08.064 BindingDB Entry DOI: 10.7270/Q22R3PXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||