

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM14073 (2S)-N-[(2S)-1-(1,3-benzothiazol-2-yl)-5-carbamimidamido-1-oxopentan-2-yl]-1-[(2R)-2-(methylamino)-3,3-diphenylpropanoyl]pyrrolidine-2-carboxamide::2-ketobenzothiazole 13::CHEMBL403768
SMILES: [#6]-[#7]-[#6@H](-[#6](-c1ccccc1)-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-c1nc2ccccc2s1
InChI Key: InChIKey=CWBJELZFIFDKMX-QXTIUPHPSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM14073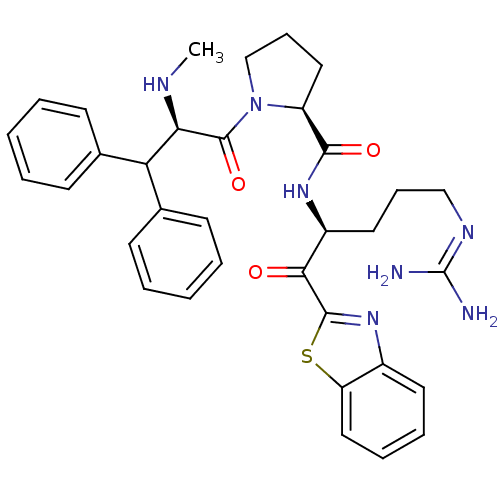 ((2S)-N-[(2S)-1-(1,3-benzothiazol-2-yl)-5-carbamimi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.000650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Binding affinity to human thrombin | J Med Chem 47: 769-87 (2004) Article DOI: 10.1021/jm030493t BindingDB Entry DOI: 10.7270/Q2251HN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14073 ((2S)-N-[(2S)-1-(1,3-benzothiazol-2-yl)-5-carbamimi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.000650 | -17.3 | 4.5 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14073 ((2S)-N-[(2S)-1-(1,3-benzothiazol-2-yl)-5-carbamimi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.000650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human alpha-thrombin | Bioorg Med Chem 16: 1562-95 (2008) Article DOI: 10.1016/j.bmc.2007.11.015 BindingDB Entry DOI: 10.7270/Q21J9BNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypsin (Bos taurus (bovine)) | BDBM14073 ((2S)-N-[(2S)-1-(1,3-benzothiazol-2-yl)-5-carbamimi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Trypsin-catalyzed hydrolysis rates were measured spectrophotometrically using bovine trypsin, a chromogenic substrate in aqueous buffer, and a microp... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14073 ((2S)-N-[(2S)-1-(1,3-benzothiazol-2-yl)-5-carbamimi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Binding activity of the compound against human alpha thrombin | J Med Chem 47: 769-87 (2004) Article DOI: 10.1021/jm030493t BindingDB Entry DOI: 10.7270/Q2251HN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||