 Found 11 hits for monomerid = 14156
Found 11 hits for monomerid = 14156 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM14156
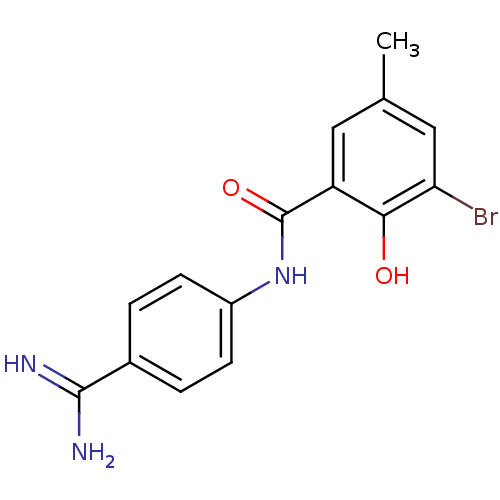
(3-bromo-N-(4-carbamimidoylphenyl)-2-hydroxy-5-meth...)Show InChI InChI=1S/C15H14BrN3O2/c1-8-6-11(13(20)12(16)7-8)15(21)19-10-4-2-9(3-5-10)14(17)18/h2-7,20H,1H3,(H3,17,18)(H,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 77 | -9.60 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Axys Pharmaceutical
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
Chem Biol 8: 1107-21 (2001)
Article DOI: 10.1016/S1074-5521(01)00084-9
BindingDB Entry DOI: 10.7270/Q2S75DKN |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM14156

(3-bromo-N-(4-carbamimidoylphenyl)-2-hydroxy-5-meth...)Show InChI InChI=1S/C15H14BrN3O2/c1-8-6-11(13(20)12(16)7-8)15(21)19-10-4-2-9(3-5-10)14(17)18/h2-7,20H,1H3,(H3,17,18)(H,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of urokinase-type plasminogen activator (microPa) |
Bioorg Med Chem Lett 12: 2023-6 (2002)
BindingDB Entry DOI: 10.7270/Q2ZG6RK4 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM14156

(3-bromo-N-(4-carbamimidoylphenyl)-2-hydroxy-5-meth...)Show InChI InChI=1S/C15H14BrN3O2/c1-8-6-11(13(20)12(16)7-8)15(21)19-10-4-2-9(3-5-10)14(17)18/h2-7,20H,1H3,(H3,17,18)(H,19,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceutical
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
Chem Biol 8: 1107-21 (2001)
Article DOI: 10.1016/S1074-5521(01)00084-9
BindingDB Entry DOI: 10.7270/Q2S75DKN |
More data for this
Ligand-Target Pair | |
Trypsin
(Bos taurus (bovine)) | BDBM14156

(3-bromo-N-(4-carbamimidoylphenyl)-2-hydroxy-5-meth...)Show InChI InChI=1S/C15H14BrN3O2/c1-8-6-11(13(20)12(16)7-8)15(21)19-10-4-2-9(3-5-10)14(17)18/h2-7,20H,1H3,(H3,17,18)(H,19,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceutical
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
Chem Biol 8: 1107-21 (2001)
Article DOI: 10.1016/S1074-5521(01)00084-9
BindingDB Entry DOI: 10.7270/Q2S75DKN |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM14156

(3-bromo-N-(4-carbamimidoylphenyl)-2-hydroxy-5-meth...)Show InChI InChI=1S/C15H14BrN3O2/c1-8-6-11(13(20)12(16)7-8)15(21)19-10-4-2-9(3-5-10)14(17)18/h2-7,20H,1H3,(H3,17,18)(H,19,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 12: 2023-6 (2002)
BindingDB Entry DOI: 10.7270/Q2ZG6RK4 |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM14156

(3-bromo-N-(4-carbamimidoylphenyl)-2-hydroxy-5-meth...)Show InChI InChI=1S/C15H14BrN3O2/c1-8-6-11(13(20)12(16)7-8)15(21)19-10-4-2-9(3-5-10)14(17)18/h2-7,20H,1H3,(H3,17,18)(H,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceutical
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
Chem Biol 8: 1107-21 (2001)
Article DOI: 10.1016/S1074-5521(01)00084-9
BindingDB Entry DOI: 10.7270/Q2S75DKN |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM14156

(3-bromo-N-(4-carbamimidoylphenyl)-2-hydroxy-5-meth...)Show InChI InChI=1S/C15H14BrN3O2/c1-8-6-11(13(20)12(16)7-8)15(21)19-10-4-2-9(3-5-10)14(17)18/h2-7,20H,1H3,(H3,17,18)(H,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Activation of plasminogen |
Bioorg Med Chem Lett 12: 2023-6 (2002)
BindingDB Entry DOI: 10.7270/Q2ZG6RK4 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM14156

(3-bromo-N-(4-carbamimidoylphenyl)-2-hydroxy-5-meth...)Show InChI InChI=1S/C15H14BrN3O2/c1-8-6-11(13(20)12(16)7-8)15(21)19-10-4-2-9(3-5-10)14(17)18/h2-7,20H,1H3,(H3,17,18)(H,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceutical
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
Chem Biol 8: 1107-21 (2001)
Article DOI: 10.1016/S1074-5521(01)00084-9
BindingDB Entry DOI: 10.7270/Q2S75DKN |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM14156

(3-bromo-N-(4-carbamimidoylphenyl)-2-hydroxy-5-meth...)Show InChI InChI=1S/C15H14BrN3O2/c1-8-6-11(13(20)12(16)7-8)15(21)19-10-4-2-9(3-5-10)14(17)18/h2-7,20H,1H3,(H3,17,18)(H,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of coagulation factor X |
Bioorg Med Chem Lett 12: 2023-6 (2002)
BindingDB Entry DOI: 10.7270/Q2ZG6RK4 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM14156

(3-bromo-N-(4-carbamimidoylphenyl)-2-hydroxy-5-meth...)Show InChI InChI=1S/C15H14BrN3O2/c1-8-6-11(13(20)12(16)7-8)15(21)19-10-4-2-9(3-5-10)14(17)18/h2-7,20H,1H3,(H3,17,18)(H,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of plasmin |
Bioorg Med Chem Lett 12: 2023-6 (2002)
BindingDB Entry DOI: 10.7270/Q2ZG6RK4 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM14156

(3-bromo-N-(4-carbamimidoylphenyl)-2-hydroxy-5-meth...)Show InChI InChI=1S/C15H14BrN3O2/c1-8-6-11(13(20)12(16)7-8)15(21)19-10-4-2-9(3-5-10)14(17)18/h2-7,20H,1H3,(H3,17,18)(H,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceutical
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
Chem Biol 8: 1107-21 (2001)
Article DOI: 10.1016/S1074-5521(01)00084-9
BindingDB Entry DOI: 10.7270/Q2S75DKN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data