

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM141763 US8921397, 5
SMILES: CCC(=O)N[C@H]1CC[C@H](CCN2CCC(CC2)c2coc3ccccc23)CC1
InChI Key: InChIKey=MUNJPNWHOINDKG-KESTWPANSA-N
Data: 3 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM141763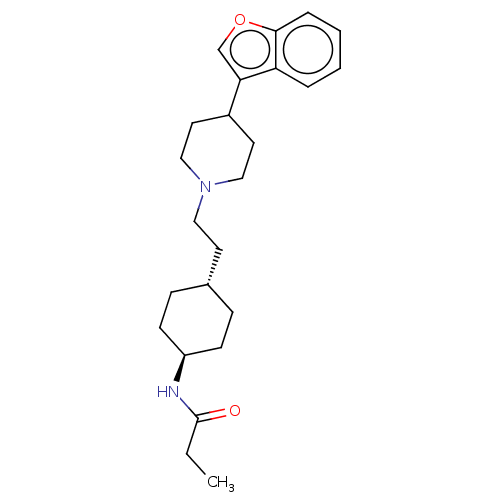 (US8921397, 5) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description liquots of membrane preparations were thawed at RT, resuspended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KC1... | US Patent US8921397 (2014) BindingDB Entry DOI: 10.7270/Q2VQ31C3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM141763 (US8921397, 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 4.52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description liquots of membrane preparations were thawed at RT, resuspended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KC1... | US Patent US8921397 (2014) BindingDB Entry DOI: 10.7270/Q2VQ31C3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM141763 (US8921397, 5) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 48.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description liquots of membrane preparations were thawed at RT, resuspended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KC1... | US Patent US8921397 (2014) BindingDB Entry DOI: 10.7270/Q2VQ31C3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||