

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: COc1cnc(cn1)C(=O)Nc1cc(F)c(F)c(c1)[C@]1(C)CS(=O)(=O)N(C)C(=N)N1
InChI Key: InChIKey=QGPBROSNFKNVFL-KRWDZBQOSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM143222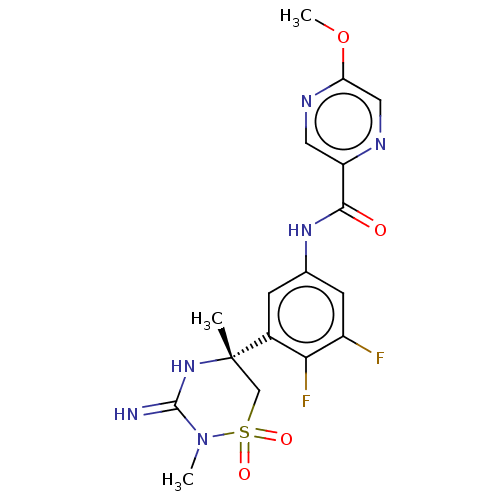 (US8940748, 40di | US9029362, 40di | US9687494, 40d...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.05 | n/a | n/a | n/a | n/a | n/a | n/a | 5.01 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The protocol that was used to determine the recited values isdescribed as follows.BACE1 HTRF FRET AssayReagentsNa+-Acetate pH 5.01% Brij-35GlycerolDi... | US Patent US8940748 (2015) BindingDB Entry DOI: 10.7270/Q2ZC81MM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM143222 (US8940748, 40di | US9029362, 40di | US9687494, 40d...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.05 | -12.4 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description A homogeneous time-resolved FRET assay was used to determine IC50 values for inhibitors of the soluble human BACE1 catalytic domain. This assay monit... | US Patent US9029362 (2015) BindingDB Entry DOI: 10.7270/Q2X63KP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM143222 (US8940748, 40di | US9029362, 40di | US9687494, 40d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.15 | -12.0 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Inhibitor IC50s at purified human autoBACE-2 were determined in a time-resolved endpoint proteolysis assay that measures hydrolysis of the QSY7-EISEV... | US Patent US9029362 (2015) BindingDB Entry DOI: 10.7270/Q2X63KP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM143222 (US8940748, 40di | US9029362, 40di | US9687494, 40d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.15 | -12.0 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Inhibitor IC50s at purified human autoBACE-2 were determined in a time-resolved endpoint proteolysis assay that measures hydrolysis of the QSY7-EISEV... | US Patent US8940748 (2015) BindingDB Entry DOI: 10.7270/Q2ZC81MM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM143222 (US8940748, 40di | US9029362, 40di | US9687494, 40d...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description This assay monitored the increase of 620 nm fluorescence that resulted from BACE1 cleavage of an APPswedish APPswe mutant peptide FRET substrate (QSY... | US Patent US9687494 (2017) BindingDB Entry DOI: 10.7270/Q2CJ8BN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM143222 (US8940748, 40di | US9029362, 40di | US9687494, 40d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description This assay monitored the increase of 620 nm fluorescence that resulted from BACE1 cleavage of an APPswedish APPswe mutant peptide FRET substrate (QSY... | US Patent US9687494 (2017) BindingDB Entry DOI: 10.7270/Q2CJ8BN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||