

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM144744 US8952128, 6B
SMILES: C[C@@H](O)[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](CCCCNC(=O)[C@H](Cc2cc(F)cc(F)c2)NC1=O)NCCSC[C@@H]1C[C@H]2[C@@H](Cc3c[nH]c4cccc2c34)N(C)C1
InChI Key:
Data: 2 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM144744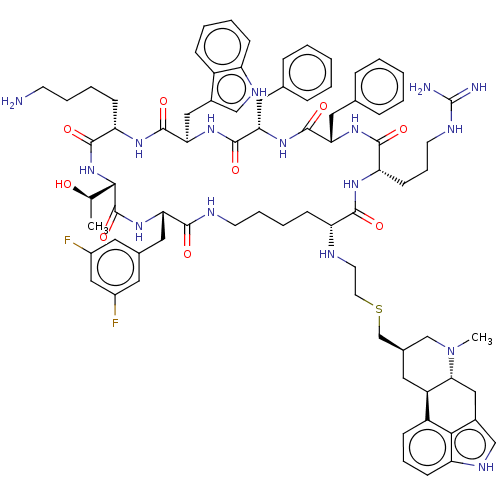 (US8952128, 6B) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.25 | -13.1 | n/a | n/a | n/a | n/a | n/a | 7.6 | 25 |
Ipsen Pharma S.A.S. US Patent | Assay Description Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w... | US Patent US8952128 (2015) BindingDB Entry DOI: 10.7270/Q2057DNG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM144744 (US8952128, 6B) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 25.4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.6 | n/a |
Ipsen Pharma S.A.S. US Patent | Assay Description The affinity of a test compound for the human dopamine receptor subtype hDRD2 was determined by radioligand binding assays in CHO-K1 cells stably tra... | US Patent US8952128 (2015) BindingDB Entry DOI: 10.7270/Q2057DNG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||