 Found 10 hits for monomerid = 14486
Found 10 hits for monomerid = 14486 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Adenosine Kinase (AK)
(Rattus norvegicus (rat)) | BDBM14486
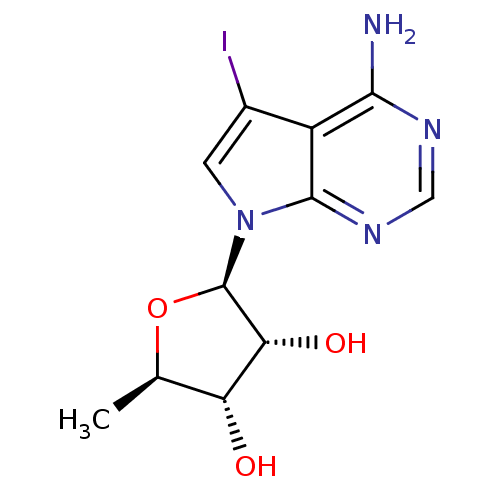
((2R,3R,4S,5R)-2-(2-amino-9-iodo-3,5,7-triazabicycl...)Show SMILES C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cc(I)c2c(N)ncnc12 |r| Show InChI InChI=1S/C11H13IN4O3/c1-4-7(17)8(18)11(19-4)16-2-5(12)6-9(13)14-3-15-10(6)16/h2-4,7-8,11,17-18H,1H3,(H2,13,14,15)/t4-,7-,8-,11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Abbott Laboratories
| Assay Description
ADK activity was monitored by a radiochemical assay, which measures the conversion of radioactive Ado ([U-14C] adenosine or [2-3H] adenosine) to AMP ... |
J Med Chem 49: 6726-31 (2006)
Article DOI: 10.1021/jm060189a
BindingDB Entry DOI: 10.7270/Q2QC01R6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine kinase
(Homo sapiens (Human)) | BDBM14486

((2R,3R,4S,5R)-2-(2-amino-9-iodo-3,5,7-triazabicycl...)Show SMILES C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cc(I)c2c(N)ncnc12 |r| Show InChI InChI=1S/C11H13IN4O3/c1-4-7(17)8(18)11(19-4)16-2-5(12)6-9(13)14-3-15-10(6)16/h2-4,7-8,11,17-18H,1H3,(H2,13,14,15)/t4-,7-,8-,11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Metabasis Therapeutics Inc
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human adenosine kinase |
J Med Chem 43: 2883-93 (2000)
BindingDB Entry DOI: 10.7270/Q2XG9QCV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine Kinase (AK)
(Rattus norvegicus (rat)) | BDBM14486

((2R,3R,4S,5R)-2-(2-amino-9-iodo-3,5,7-triazabicycl...)Show SMILES C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cc(I)c2c(N)ncnc12 |r| Show InChI InChI=1S/C11H13IN4O3/c1-4-7(17)8(18)11(19-4)16-2-5(12)6-9(13)14-3-15-10(6)16/h2-4,7-8,11,17-18H,1H3,(H2,13,14,15)/t4-,7-,8-,11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of Adenosine kinase (AK) |
J Med Chem 44: 2133-8 (2001)
BindingDB Entry DOI: 10.7270/Q25D8R47 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine kinase
(Homo sapiens (Human)) | BDBM14486

((2R,3R,4S,5R)-2-(2-amino-9-iodo-3,5,7-triazabicycl...)Show SMILES C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cc(I)c2c(N)ncnc12 |r| Show InChI InChI=1S/C11H13IN4O3/c1-4-7(17)8(18)11(19-4)16-2-5(12)6-9(13)14-3-15-10(6)16/h2-4,7-8,11,17-18H,1H3,(H2,13,14,15)/t4-,7-,8-,11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| n/a | n/a | 22.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of adenosine phosphorylation in confluent IMR-32 (human neuroblastoma) cells. |
J Med Chem 44: 2133-8 (2001)
BindingDB Entry DOI: 10.7270/Q25D8R47 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine kinase
(Homo sapiens (Human)) | BDBM14486

((2R,3R,4S,5R)-2-(2-amino-9-iodo-3,5,7-triazabicycl...)Show SMILES C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cc(I)c2c(N)ncnc12 |r| Show InChI InChI=1S/C11H13IN4O3/c1-4-7(17)8(18)11(19-4)16-2-5(12)6-9(13)14-3-15-10(6)16/h2-4,7-8,11,17-18H,1H3,(H2,13,14,15)/t4-,7-,8-,11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Inhibition of human adenosine kinase assessed as reduction in conversion of adenosine to AMP |
J Med Chem 59: 6860-77 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00689 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine kinase
(Homo sapiens (Human)) | BDBM14486

((2R,3R,4S,5R)-2-(2-amino-9-iodo-3,5,7-triazabicycl...)Show SMILES C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cc(I)c2c(N)ncnc12 |r| Show InChI InChI=1S/C11H13IN4O3/c1-4-7(17)8(18)11(19-4)16-2-5(12)6-9(13)14-3-15-10(6)16/h2-4,7-8,11,17-18H,1H3,(H2,13,14,15)/t4-,7-,8-,11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Jadavpur University
Curated by ChEMBL
| Assay Description
Inhibition of human adenosine kinase activity |
Bioorg Med Chem Lett 12: 899-902 (2002)
BindingDB Entry DOI: 10.7270/Q2NP23QT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine kinase
(Homo sapiens (Human)) | BDBM14486

((2R,3R,4S,5R)-2-(2-amino-9-iodo-3,5,7-triazabicycl...)Show SMILES C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cc(I)c2c(N)ncnc12 |r| Show InChI InChI=1S/C11H13IN4O3/c1-4-7(17)8(18)11(19-4)16-2-5(12)6-9(13)14-3-15-10(6)16/h2-4,7-8,11,17-18H,1H3,(H2,13,14,15)/t4-,7-,8-,11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Metabasis Therapeutics Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human recombinant adenosine kinase using [14C]-AMP as radioligand |
J Med Chem 48: 6430-41 (2005)
Article DOI: 10.1021/jm0503650
BindingDB Entry DOI: 10.7270/Q2DV1JFS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine kinase
(Homo sapiens (Human)) | BDBM14486

((2R,3R,4S,5R)-2-(2-amino-9-iodo-3,5,7-triazabicycl...)Show SMILES C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cc(I)c2c(N)ncnc12 |r| Show InChI InChI=1S/C11H13IN4O3/c1-4-7(17)8(18)11(19-4)16-2-5(12)6-9(13)14-3-15-10(6)16/h2-4,7-8,11,17-18H,1H3,(H2,13,14,15)/t4-,7-,8-,11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 8.91 | n/a | n/a | n/a | n/a | n/a | n/a |
Vigo University
Curated by ChEMBL
| Assay Description
Concentration required for 50% inhibition of the adenosine kinase (AK) activity. |
Bioorg Med Chem Lett 14: 3077-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.040
BindingDB Entry DOI: 10.7270/Q2CZ38BM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine kinase
(Homo sapiens (Human)) | BDBM14486

((2R,3R,4S,5R)-2-(2-amino-9-iodo-3,5,7-triazabicycl...)Show SMILES C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cc(I)c2c(N)ncnc12 |r| Show InChI InChI=1S/C11H13IN4O3/c1-4-7(17)8(18)11(19-4)16-2-5(12)6-9(13)14-3-15-10(6)16/h2-4,7-8,11,17-18H,1H3,(H2,13,14,15)/t4-,7-,8-,11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
| n/a | n/a | 9.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of adenosine kinase (unknown origin) |
Citation and Details
Article DOI: 10.1007/s00044-004-0048-0
BindingDB Entry DOI: 10.7270/Q2XG9V11 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine Kinase (AK)
(Rattus norvegicus (rat)) | BDBM14486

((2R,3R,4S,5R)-2-(2-amino-9-iodo-3,5,7-triazabicycl...)Show SMILES C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cc(I)c2c(N)ncnc12 |r| Show InChI InChI=1S/C11H13IN4O3/c1-4-7(17)8(18)11(19-4)16-2-5(12)6-9(13)14-3-15-10(6)16/h2-4,7-8,11,17-18H,1H3,(H2,13,14,15)/t4-,7-,8-,11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of Adenosine kinase of rat brain cytosol. |
Bioorg Med Chem Lett 11: 2419-22 (2001)
BindingDB Entry DOI: 10.7270/Q2M32V1S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data