

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM14593 3-amino-5-[1-({[(4-carbamimidoylphenyl)methyl]carbamoyl}methyl)-3-chloro-6-oxo-5-(propan-2-ylamino)-1,6-dihydropyrazin-2-yl]benzoic acid::CHEMBL77076::Pyrazinone Analog 34
SMILES: CC(C)Nc1nc(Cl)c(-c2cc(N)cc(c2)C(O)=O)n(CC(=O)NCc2ccc(cc2)C(N)=N)c1=O
InChI Key: InChIKey=XDNNQWBDYFHHSY-UHFFFAOYSA-N
Data: 9 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM14593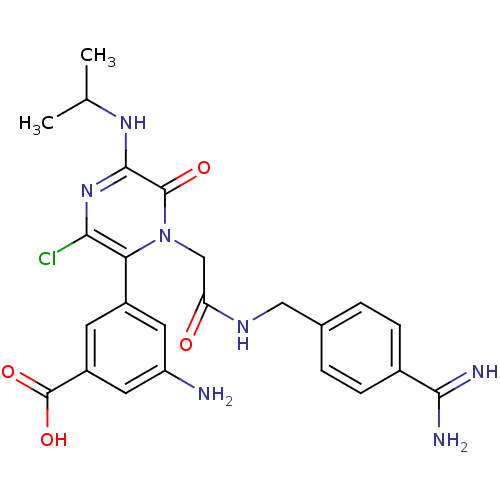 (3-amino-5-[1-({[(4-carbamimidoylphenyl)methyl]carb...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Corporation | Assay Description Human thrombin and substrate were added to a 96-well assay plate containing inhibitor in reaction buffer. The rate of hydrolysis of the substrate was... | J Med Chem 46: 4050-62 (2003) Article DOI: 10.1021/jm030131l BindingDB Entry DOI: 10.7270/Q2FT8J9M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM14593 (3-amino-5-[1-({[(4-carbamimidoylphenyl)methyl]carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Corporation | Assay Description Human Factor Xa and substrate were added to a 96-well assay plate containing inhibitor in reaction buffer. The rate of hydrolysis of the substrate wa... | J Med Chem 46: 4050-62 (2003) Article DOI: 10.1021/jm030131l BindingDB Entry DOI: 10.7270/Q2FT8J9M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM14593 (3-amino-5-[1-({[(4-carbamimidoylphenyl)methyl]carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Corporation | Assay Description Recombinant human Factor VIIa and soluble tissue factor were added to a 96-well assay plate containing substrate and inhibitor in reaction buffer. Th... | J Med Chem 46: 4050-62 (2003) Article DOI: 10.1021/jm030131l BindingDB Entry DOI: 10.7270/Q2FT8J9M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM14593 (3-amino-5-[1-({[(4-carbamimidoylphenyl)methyl]carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Corporation Curated by ChEMBL | Assay Description Inhibitory activity of the compound against coagulation factor X | Bioorg Med Chem Lett 13: 2319-25 (2003) BindingDB Entry DOI: 10.7270/Q2G16068 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor III/Factor VIIa (fVIIa) (Homo sapiens (Human)) | BDBM14593 (3-amino-5-[1-({[(4-carbamimidoylphenyl)methyl]carb...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Hefei University of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant soluble tissue factor (unknown origin)/recombinant human factor 7a using N-methylsulfonyl-D-phe-gly arg-p-nitroaniline as s... | Eur J Med Chem 146: 299-317 (2018) Article DOI: 10.1016/j.ejmech.2018.01.067 BindingDB Entry DOI: 10.7270/Q2251MTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14593 (3-amino-5-[1-({[(4-carbamimidoylphenyl)methyl]carb...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Corporation Curated by ChEMBL | Assay Description Inhibitory activity of the compound against Thrombin | Bioorg Med Chem Lett 13: 2319-25 (2003) BindingDB Entry DOI: 10.7270/Q2G16068 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM14593 (3-amino-5-[1-({[(4-carbamimidoylphenyl)methyl]carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hefei University of Technology Curated by ChEMBL | Assay Description Inhibition of human factor 10a using N-alpha-benzyloxy-carbonyl-D-arginyl-L-glycyl-L-arginine-p-nitroaniline-dihydrochloride as substrate after 60 mi... | Eur J Med Chem 146: 299-317 (2018) Article DOI: 10.1016/j.ejmech.2018.01.067 BindingDB Entry DOI: 10.7270/Q2251MTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14593 (3-amino-5-[1-({[(4-carbamimidoylphenyl)methyl]carb...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hefei University of Technology Curated by ChEMBL | Assay Description Inhibition of human thrombin using H-D-phenylalanyl-L-pipecolyl-L-arginine-p-nitroaniline dihydrochloride as substrate after 60 mins | Eur J Med Chem 146: 299-317 (2018) Article DOI: 10.1016/j.ejmech.2018.01.067 BindingDB Entry DOI: 10.7270/Q2251MTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypsin-1 (Homo sapiens (Human)) | BDBM14593 (3-amino-5-[1-({[(4-carbamimidoylphenyl)methyl]carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Corporation Curated by ChEMBL | Assay Description Inhibitory activity of the compound against trypsin | Bioorg Med Chem Lett 13: 2319-25 (2003) BindingDB Entry DOI: 10.7270/Q2G16068 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||