

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM15188 5-{5-[(1,3-dioxo-2,3-dihydro-1H-inden-2-ylidene)methyl]furan-2-yl}-2-hydroxybenzoic acid::CHEMBL175828::compound 3::compound 5378650
SMILES: [#8]-[#6](=O)-c1cc(ccc1-[#8])-c1ccc(\[#6]=[#6]-2/[#6](=O)-c3ccccc3-[#6]-2=O)o1
InChI Key: InChIKey=MIMODCWGTPVIBH-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM15188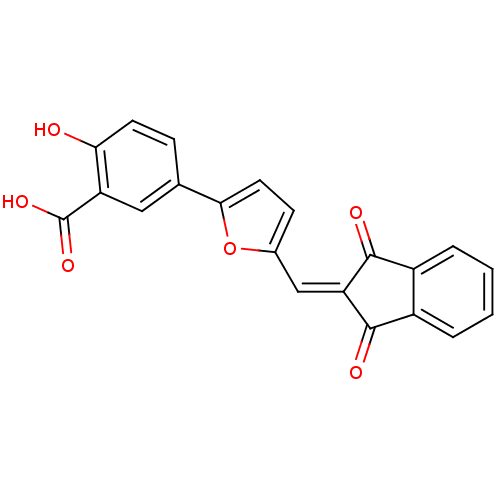 (5-{5-[(1,3-dioxo-2,3-dihydro-1H-inden-2-ylidene)me...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.08E+4 | -6.38 | 2.51E+4 | n/a | n/a | n/a | n/a | 7.5 | 25 |
The Burnham Institute | Assay Description The Z-LYTE assay (Invitrogen Corporation) employs a FRET-based, coupled-enzyme format and is based on the differential sensitivity of phosphorylated ... | J Med Chem 48: 2278-81 (2005) Article DOI: 10.1021/jm048962u BindingDB Entry DOI: 10.7270/Q2KD1W4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 reverse transcriptase (Human immunodeficiency virus 1) | BDBM15188 (5-{5-[(1,3-dioxo-2,3-dihydro-1H-inden-2-ylidene)me...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California Curated by ChEMBL | Assay Description Inhibitory concentration against human immuno deficiency virus type 1 integrase (3'-processing) | J Med Chem 48: 111-20 (2005) Article DOI: 10.1021/jm0496077 BindingDB Entry DOI: 10.7270/Q28S4PD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 reverse transcriptase (Human immunodeficiency virus 1) | BDBM15188 (5-{5-[(1,3-dioxo-2,3-dihydro-1H-inden-2-ylidene)me...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California Curated by ChEMBL | Assay Description Inhibitory concentration against human immuno deficiency virus type 1 integrase (Strand Transfer) | J Med Chem 48: 111-20 (2005) Article DOI: 10.1021/jm0496077 BindingDB Entry DOI: 10.7270/Q28S4PD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||