

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: CCc1cc2c(ncnc2s1)N1CCN(CC1)C1=NCC(C)(C)S1
InChI Key: InChIKey=PLPPLEOZEGMVKB-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Menin (Homo sapiens (Human)) | BDBM152230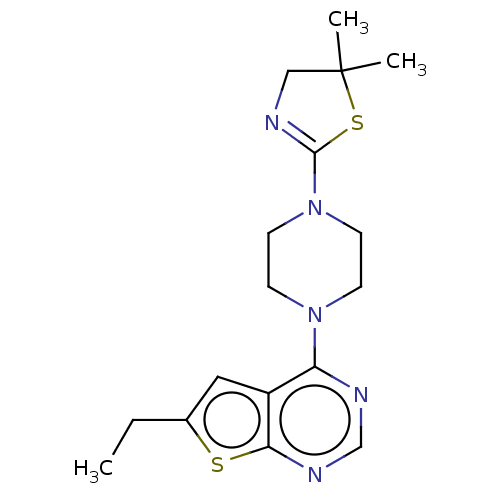 (US8993552, 65) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of N-terminal thioredoxin His6-tagged full-length human menin expressed in Escherichia coli Rosetta (DE3) cells assessed as reduction in m... | J Med Chem 58: 7465-74 (2015) BindingDB Entry DOI: 10.7270/Q25H7J2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Menin (Homo sapiens (Human)) | BDBM152230 (US8993552, 65) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of The University of Michigan; University of Virginia Patent Foundation US Patent | Assay Description NMR spectroscopy validation of lead compounds. In embodiments of the present invention, and during development thereof, NMR spectroscopy: saturation ... | US Patent US8993552 (2015) BindingDB Entry DOI: 10.7270/Q2V123HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||