

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM15297 CHEMBL34431::Cilostamide::N-Cyclohexyl-N-methyl-4-(2-oxo-1,2-dihydro-quinolin-6-yloxy)-butyramide::N-cyclohexyl-N-methyl-4-[(2-oxo-1,2-dihydroquinolin-6-yl)oxy]butanamide
SMILES: CN(C1CCCCC1)C(=O)CCCOc1ccc2[nH]c(=O)ccc2c1
InChI Key: InChIKey=UIAYVIIHMORPSJ-UHFFFAOYSA-N
Data: 11 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phosphodiesterase Type 3 (PDE3B) (Homo sapiens (Human)) | BDBM15297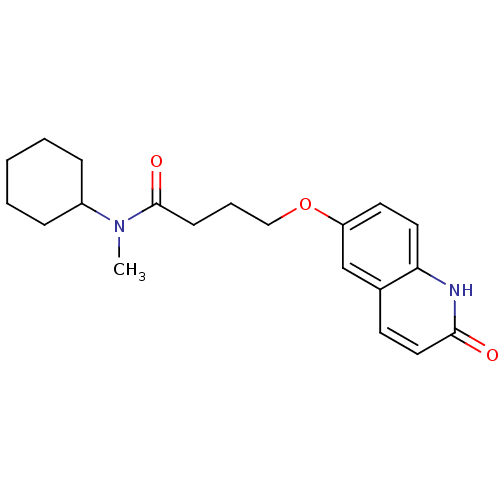 (CHEMBL34431 | Cilostamide | N-Cyclohexyl-N-methyl-...) | PDB MMDB KEGG B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Merck Research Laboratories | Assay Description PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using scintillation proximity assay (SPA). [3H]-AMP was captured by t... | Bioorg Med Chem Lett 13: 3983-7 (2003) Article DOI: 10.1016/j.bmcl.2003.08.056 BindingDB Entry DOI: 10.7270/Q2JD4V11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphodiesterase Type 3 (PDE3A) (Homo sapiens (Human)) | BDBM15297 (CHEMBL34431 | Cilostamide | N-Cyclohexyl-N-methyl-...) | PDB KEGG DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories | Assay Description PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using scintillation proximity assay (SPA). [3H]-AMP was captured by t... | Bioorg Med Chem Lett 13: 3983-7 (2003) Article DOI: 10.1016/j.bmcl.2003.08.056 BindingDB Entry DOI: 10.7270/Q2JD4V11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphodiesterase (TcrPDEC) (Trypanosoma cruzi) | BDBM15297 (CHEMBL34431 | Cilostamide | N-Cyclohexyl-N-methyl-...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 7.5 | 24 |
University of North Carolina | Assay Description Enzymatic activities were assayed using [3H] cAMP and [3H]cGMP as substrate. | J Biol Chem 287: 11788-97 (2012) Article DOI: 10.1074/jbc.M111.326777 BindingDB Entry DOI: 10.7270/Q2K9364D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphodiesterase 3 (PDE3) (Homo sapiens (Human)) | BDBM15297 (CHEMBL34431 | Cilostamide | N-Cyclohexyl-N-methyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | 24 |
University of North Carolina | Assay Description Enzymatic activities were assayed using [3H] cAMP and [3H]cGMP as substrate. | J Biol Chem 287: 11788-97 (2012) Article DOI: 10.1074/jbc.M111.326777 BindingDB Entry DOI: 10.7270/Q2K9364D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM15297 (CHEMBL34431 | Cilostamide | N-Cyclohexyl-N-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 7.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against phosphodiesterase 5 (PDE5) from human platelet | Bioorg Med Chem Lett 14: 2955-8 (2004) Article DOI: 10.1016/j.bmcl.2004.03.021 BindingDB Entry DOI: 10.7270/Q2M044W1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphodiesterase 3 (PDE3) (Homo sapiens (Human)) | BDBM15297 (CHEMBL34431 | Cilostamide | N-Cyclohexyl-N-methyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 18.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE3A | Bioorg Med Chem Lett 21: 1617-20 (2011) Article DOI: 10.1016/j.bmcl.2011.01.120 BindingDB Entry DOI: 10.7270/Q2WQ043X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphodiesterase 3 (Homo sapiens (Human)) | BDBM15297 (CHEMBL34431 | Cilostamide | N-Cyclohexyl-N-methyl-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against phosphodiesterase 3 from human platelet | Bioorg Med Chem Lett 14: 2955-8 (2004) Article DOI: 10.1016/j.bmcl.2004.03.021 BindingDB Entry DOI: 10.7270/Q2M044W1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphodiesterase 1 (Bos taurus-BOVINE) | BDBM15297 (CHEMBL34431 | Cilostamide | N-Cyclohexyl-N-methyl-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against phosphodiesterase 1 from bovine, calmodulin | Bioorg Med Chem Lett 14: 2955-8 (2004) Article DOI: 10.1016/j.bmcl.2004.03.021 BindingDB Entry DOI: 10.7270/Q2M044W1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphodiesterase 2A (Rattus norvegicus) | BDBM15297 (CHEMBL34431 | Cilostamide | N-Cyclohexyl-N-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against phosphodiesterase 2 from rat kidney | Bioorg Med Chem Lett 14: 2955-8 (2004) Article DOI: 10.1016/j.bmcl.2004.03.021 BindingDB Entry DOI: 10.7270/Q2M044W1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-inhibited 3',5'-cyclic phosphodiesterase B (Homo sapiens (Human)) | BDBM15297 (CHEMBL34431 | Cilostamide | N-Cyclohexyl-N-methyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 69.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE3B | Bioorg Med Chem Lett 21: 1617-20 (2011) Article DOI: 10.1016/j.bmcl.2011.01.120 BindingDB Entry DOI: 10.7270/Q2WQ043X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphodiesterase 4 (RAT-Rattus norvegicus) | BDBM15297 (CHEMBL34431 | Cilostamide | N-Cyclohexyl-N-methyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against phosphodiesterase 4 (PDE4) from rat kidney | Bioorg Med Chem Lett 14: 2955-8 (2004) Article DOI: 10.1016/j.bmcl.2004.03.021 BindingDB Entry DOI: 10.7270/Q2M044W1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||