

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM155253 US10098888, Compound 1::US9006242, 1
SMILES: CCN([C@H]1CC[C@@H](CC1)N(C)C)c1cc(cc(C(=O)NCc2c(C)cc(C)[nH]c2=O)c1C)-c1ccc(OCCOC)cc1
InChI Key:
Data: 12 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 1A1 (Homo sapiens (Human)) | BDBM155253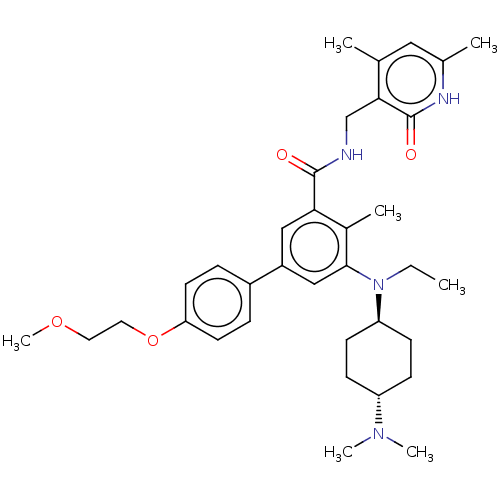 (US10098888, Compound 1 | US9006242, 1) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Epizyme, Inc. US Patent | Assay Description The potential inhibition of enzyme activities of human cytochromes P450 (CYP) of Compound 1, 2, or 105 was evaluated using pooled human liver microso... | US Patent US9006242 (2015) BindingDB Entry DOI: 10.7270/Q2222SHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM155253 (US10098888, Compound 1 | US9006242, 1) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Epizyme, Inc. US Patent | Assay Description The potential inhibition of enzyme activities of human cytochromes P450 (CYP) of Compound 1, 2, or 105 was evaluated using pooled human liver microso... | US Patent US9006242 (2015) BindingDB Entry DOI: 10.7270/Q2222SHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM155253 (US10098888, Compound 1 | US9006242, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Epizyme, Inc. US Patent | Assay Description The potential inhibition of enzyme activities of human cytochromes P450 (CYP) of Compound 1, 2, or 105 was evaluated using pooled human liver microso... | US Patent US9006242 (2015) BindingDB Entry DOI: 10.7270/Q2222SHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM155253 (US10098888, Compound 1 | US9006242, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 8.96E+4 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Epizyme, Inc. US Patent | Assay Description The potential inhibition of enzyme activities of human cytochromes P450 (CYP) of Compound 1, 2, or 105 was evaluated using pooled human liver microso... | US Patent US9006242 (2015) BindingDB Entry DOI: 10.7270/Q2222SHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM155253 (US10098888, Compound 1 | US9006242, 1) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.29E+4 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Epizyme, Inc. US Patent | Assay Description The potential inhibition of enzyme activities of human cytochromes P450 (CYP) of Compound 1, 2, or 105 was evaluated using pooled human liver microso... | US Patent US9006242 (2015) BindingDB Entry DOI: 10.7270/Q2222SHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM155253 (US10098888, Compound 1 | US9006242, 1) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 22.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description The potential inhibition of enzyme activities of human cytochromes P450 (CYP) of Compound 1, 2, or 105 was evaluated using pooled human liver microso... | US Patent US10098888 (2018) BindingDB Entry DOI: 10.7270/Q2BK1FDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| H3K27me2 (Homo sapiens (Human)) | BDBM155253 (US10098888, Compound 1 | US9006242, 1) | GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description General Materials. S-adenosylmethionine (SAM), S-adenosylhomocyteine (SAH), bicine, KCI, Tween20, dimethylsulfoxide (DMSO) and bovine skin gelatin (B... | US Patent US10098888 (2018) BindingDB Entry DOI: 10.7270/Q2BK1FDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A (Homo sapiens (Human)) | BDBM155253 (US10098888, Compound 1 | US9006242, 1) | PDB MMDB Reactome pathway KEGG B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description The potential inhibition of enzyme activities of human cytochromes P450 (CYP) of Compound 1, 2, or 105 was evaluated using pooled human liver microso... | US Patent US10098888 (2018) BindingDB Entry DOI: 10.7270/Q2BK1FDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM155253 (US10098888, Compound 1 | US9006242, 1) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description The potential inhibition of enzyme activities of human cytochromes P450 (CYP) of Compound 1, 2, or 105 was evaluated using pooled human liver microso... | US Patent US10098888 (2018) BindingDB Entry DOI: 10.7270/Q2BK1FDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM155253 (US10098888, Compound 1 | US9006242, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description The potential inhibition of enzyme activities of human cytochromes P450 (CYP) of Compound 1, 2, or 105 was evaluated using pooled human liver microso... | US Patent US10098888 (2018) BindingDB Entry DOI: 10.7270/Q2BK1FDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM155253 (US10098888, Compound 1 | US9006242, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 89.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description The potential inhibition of enzyme activities of human cytochromes P450 (CYP) of Compound 1, 2, or 105 was evaluated using pooled human liver microso... | US Patent US10098888 (2018) BindingDB Entry DOI: 10.7270/Q2BK1FDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| H3K27me3 (Homo sapiens (Human)) | BDBM155253 (US10098888, Compound 1 | US9006242, 1) | GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description General Materials. S-adenosylmethionine (SAM), S-adenosylhomocyteine (SAH), bicine, KCI, Tween20, dimethylsulfoxide (DMSO) and bovine skin gelatin (B... | US Patent US10098888 (2018) BindingDB Entry DOI: 10.7270/Q2BK1FDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||