

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: CC(C)N1CCN(CC1)C(=O)OC1CCN(CC1)c1cc[nH]c(=O)c1
InChI Key: InChIKey=JEOAGENLLSBVFS-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM159252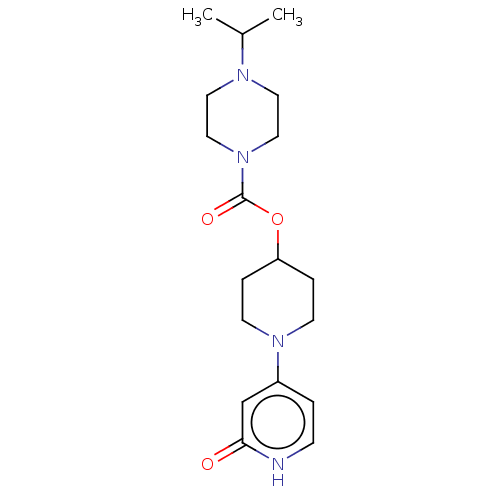 (US9034874, 2.3) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description The potency of compounds of the invention as H3 receptor antagonists can be assessed by measuring the blockade of (R)-alpha-methylhistamine-mediated ... | US Patent US9034874 (2015) BindingDB Entry DOI: 10.7270/Q2C82819 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM159252 (US9034874, 2.3) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 12 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
NOVARTIS AG US Patent | Assay Description The affinity of compounds of the invention to the H3 receptor can be assessed by measuring displacement of binding of the radioligand [3H]-N-alpha -M... | US Patent US9034874 (2015) BindingDB Entry DOI: 10.7270/Q2C82819 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||